"The regulatory sandbox that we use is similar to the regulatory sandbox used in the financial technology (fintech) sector," said Setiaji, the ministry's expert staff and chief of the digital transformation office, at a press conference in Jakarta on Thursday.
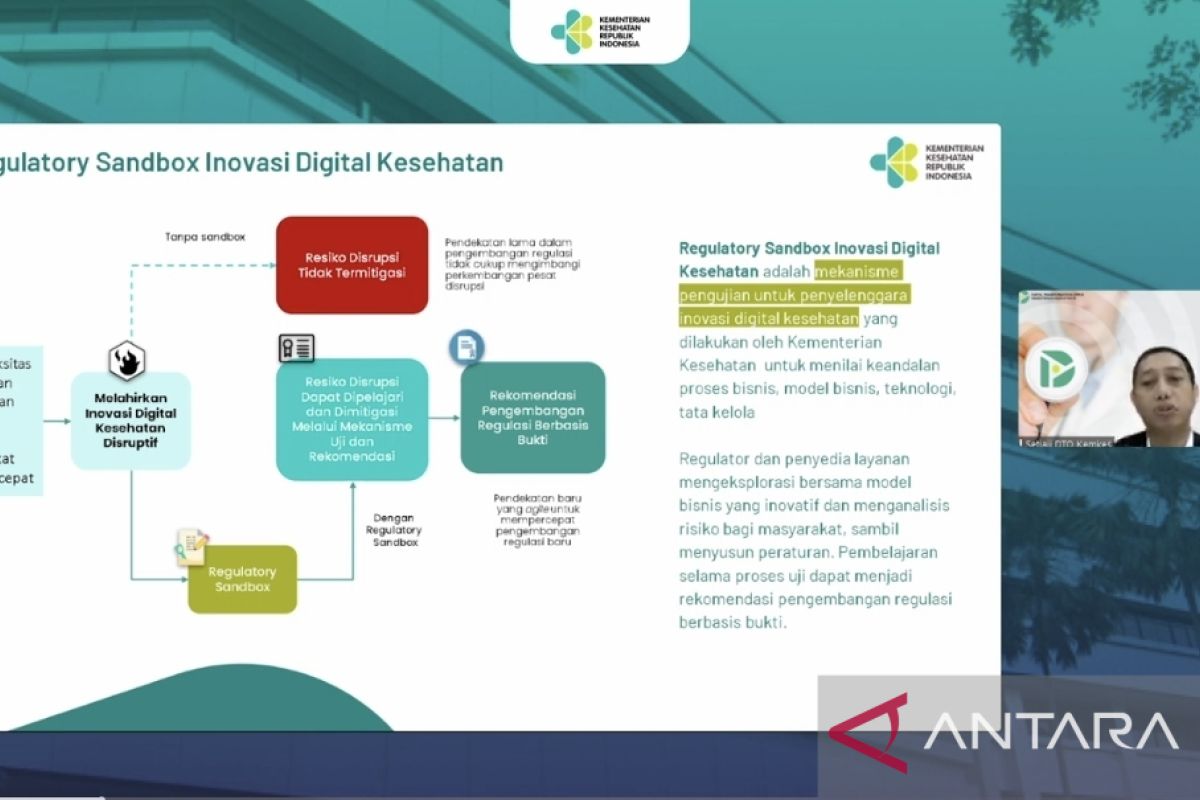
He said the regulatory sandbox is a testing mechanism for digital health innovation carried out by the Ministry of Health in collaboration with various experts in related fields.
The regulatory sandbox will assess the reliability of business processes, business models, technology, regulators' management, and service providers to jointly explore innovative business models and analyze risks to society while developing regulations that support the implementation of these technologies in the health sector, he stated.
"The learning during the test process can become a recommendation for the development of evidence-based regulations in the health sector," he remarked.
Setiaji noted that currently, the health industry, especially digitalization in the tele-health sector during COVID-19, had made a significant contribution to help people in accessing digital health services.
Based on data from the Indonesian Telemedicine Alliance (Atensi) in 2022, there were more than 17.9 million telemedicine consultation activities from 19 telemedicine companies.
The Health Ministry has also issued Decree (SK) No. HK.01.07/Menkes/1280/2023 concerning Development of Digital Innovation Through Regulatory Sandbox.
Setiaji noted that the ministry had conducted limited trials of the system on malaria and is now developing it for tele-health services.
"We have opened regulatory sandbox registration for health technology transformation on April 3 to May 5, 2023," he stated.
Setiaji explained that with the regulatory sandbox, hospitals can strengthen consumer protection and patient safety, including the personal data of service users.
For health workers, the ministry has prepared standardization regarding procedures for using the regulatory sandbox technology and strengthening protection for health workers, he noted.
Meanwhile, the regulatory sandbox will be beneficial to the pharmaceutical industry, especially health technology developers, by strengthening the industry through a mechanism for issuing temporary regulations in the form of recommendations and guidance for those who pass the supervision process, he stated.
"The government will prepare recommendations to help the implementation of this mechanism that could develop more specific policies and regulations in the field of data-based health technology," Setiaji remarked.
Related news: Endeavoring for a better health system in Indonesia
Related news: Widodo calls for building education, health facilities at IKN
Translator: Andi Firdaus, Resinta S
Editor: Anton Santoso
Copyright © ANTARA 2023