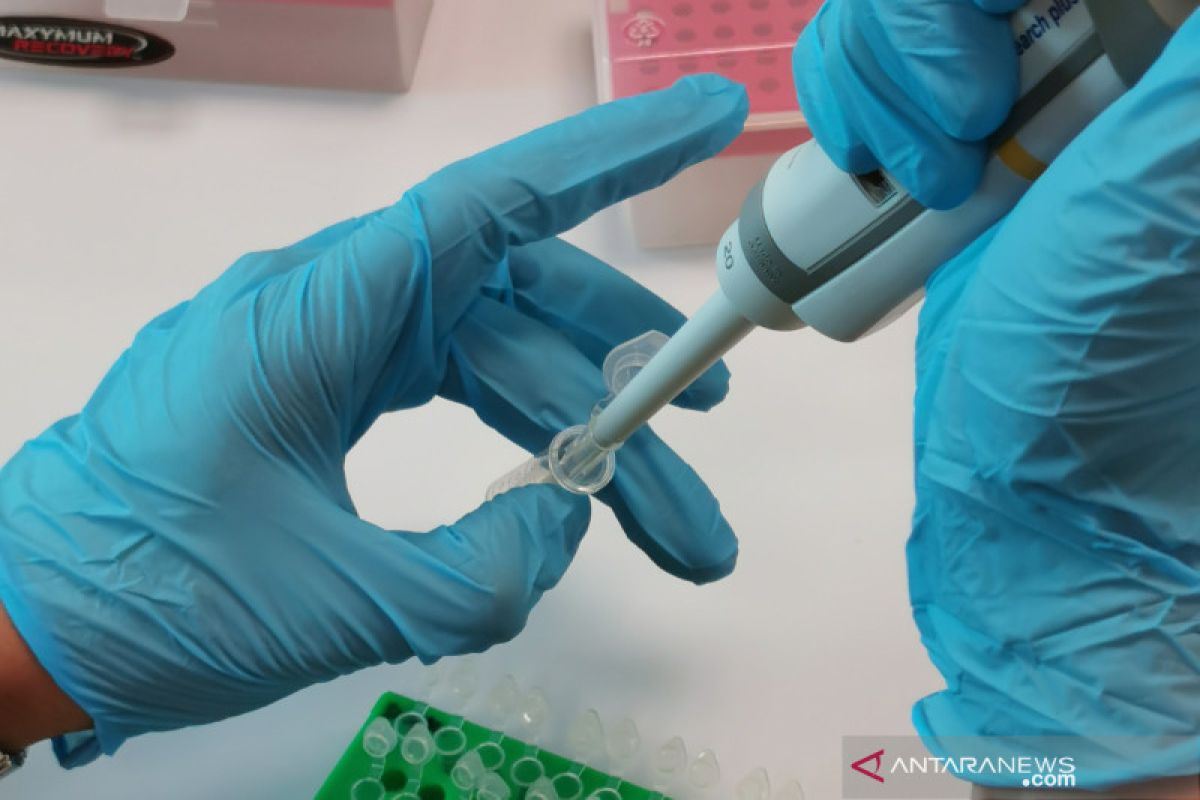
The design process and laboratory testing of the accuracy of the test kit named Nusantara TFRIC-19 was completed on Friday (4/4). The development of this test kit was carried out by Nusantics as part of the Task Force for Research and Technology Innovation for Handling COVID-19 (TFRIC19) formed by the Agency for Assessment and Application of Technology (BPPT).
CTO Nusantics Revata Utama through his statement on Sunday, explained that Nusantics designed Nusantara TFRIC-19 using SARS-CoV-2 virus genomic data which spread in Asia.
"We tested the prototype's accuracy and validation of Asian strains. As a result, Nusantara TFRIC-19 can identify COVID-19 strains, which are based on data already similar to the prediction of Indonesian strain mutations, " Revata said.
Nusantics conducted a bioinformatics analysis in the first stage by aligning the genetic sequences of the SARS-CoV-2 virus that is endemic in Asia to select target genes.
From bioinformatics analysis, Nusantics decided to design Nusantara TFRIC-19 to target two SARS-CoV-2 virus genes, namely the RdRP gene (which produces enzymes to replicate viruses when infecting human cells) and Gen N (which protects the core of RNA virus).
Then, the sensitivity of the test kit is tested using RNA isolates from abroad. Isolates from abroad are used to accelerate development, as long as local RNA isolates are not yet available.
Laboratory test results prove that Nusantara TFRIC-19 can detect the SARS-CoV-2 virus that causes COVID-19 specifically, and does not react to the genome of the SARS-CoV-1 virus or other coronaviruses.
Nusantics will then conduct laboratory tests using samples of the SARS-CoV-2 virus circulating in Indonesia. Genomic funds from the local virus will be used to complete the Nusantara TFRIC-19 prototype.
The BPPT Task Force for COVID-19 is currently still waiting to get local virus samples from the Health Research and Development Agency (Litbangkes). Completion of the Nusantara TFRIC-19 prototype is expected within 2-3 days after the sample is obtained.
"Validation with Asian virus strains shows that Nusantara TFRIC-19 is able to diagnose COVID-19 accurately. However, we will improve it by using local virus genomic information, " Nusantics CEO Sharlini Eriza Putri remarked.
"This is the reason, why local strain samples are very important. After getting local samples, we will validate Nusantara TFRIC-19 accuracy with Indonesian strains. After that, we can enter the mass production stage," Eriza continued.
"Indonesia Pasti Bisa" which was initiated by East Ventures, raised fund of Rp10 billion to support the efforts of the Research and Technology Innovation Task Force for Handling COVID-19 in developing and producing COVID-19 test kits made in Indonesia.
The test kit produced by Nusantics will be produced massively by BPPT, Bio Farma, and the Indonesia International Institute for Life Science.
Funds collected through the "Indonesia Pasti Bisa" will be used to finance the purchase of materials and raw materials to produce 100,000 qPCR test kits.
Related news: E Java governor turns official residence into COVID-19 command center
Related news: TransJakarta requires passengers to wear masks
Reporter: Arnidhya Nur, Azis Kurmala
Editor: Fardah Assegaf
Copyright © ANTARA 2020