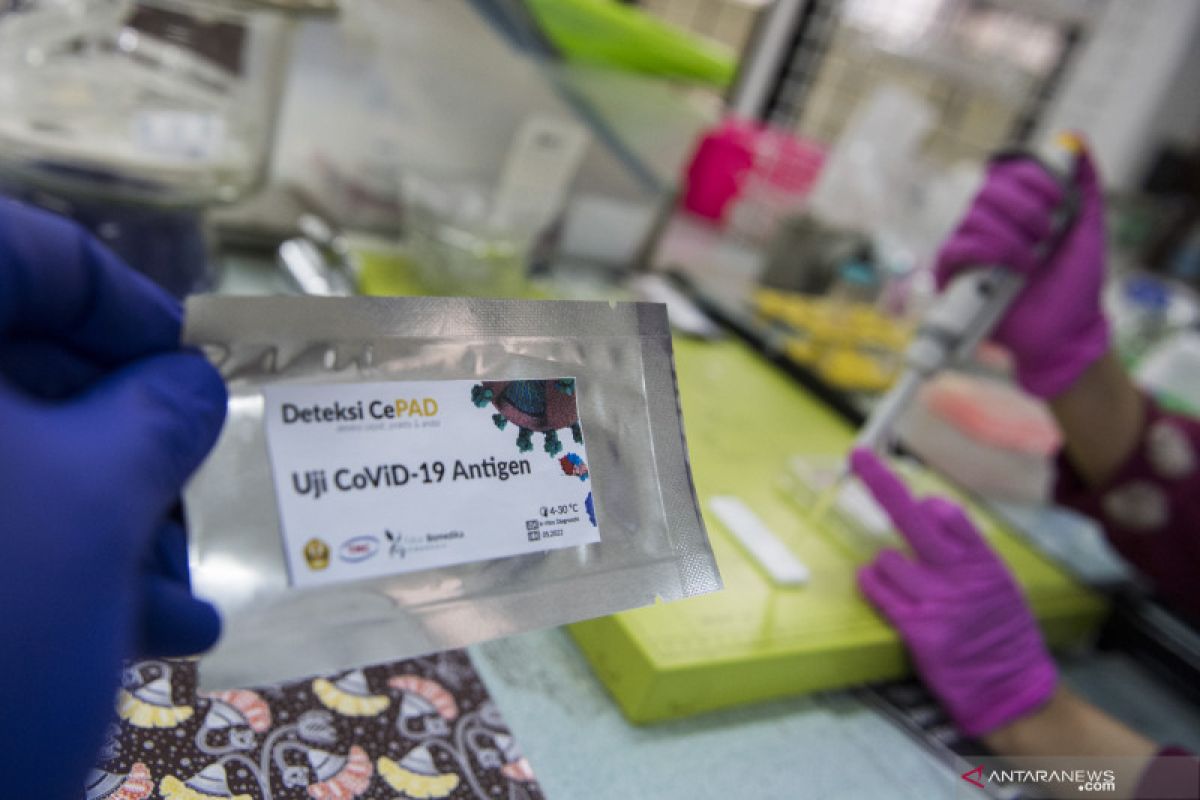
The Indonesian government will no doubt conduct (antigen-based rapid diagnostic tests). We have received a recommendation from the WHO on (the use of) antigen-based rapid diagnostic tests that have good quality.Jakarta (ANTARA) - The Indonesian government plans to use antigen-based rapid diagnostic tests to detect COVID-19, as recommended by the World Health Organization (WHO), spokesperson for the Task Force for COVID-19 Response, Wiku Adisasmito, said.
“The Indonesian government will no doubt conduct (antigen-based rapid diagnostic tests). We have received a recommendation from the WHO on (the use of) antigen-based rapid diagnostic tests that have good quality,” he said at an online press conference at the Presidential Office here on Thursday.
“The antigen-based rapid tests approved by WHO are 15-30 minutes faster, easier, and cheaper and can be used for examination at schools, university campuses, and workplaces,” he added.
The WHO plans to provide 120 million units of the antigen-based rapid tests to middle- and low-income countries. The world body has set the price of Emergency Use Listing (EUL) antigen-based rapid tests at US$5 per unit.
“We are reviewing it. We will likely use it because it has higher level of accuracy. Since it detects antigens, of course, it will detect antibodies more properly. The screening process will be conducted before diagnostic tests using real-time PCR," Adisasmito said.
The government has held talks in this regard with the WHO representative in Indonesia, he added.
Pharmaceutical majors Abbott and SD Biosensor have signed an agreement with the Bill and Melinda Gates Foundation to produce 1,290 million units of the test. The agreement will allow the companies to take the tests to 133 countries, including Latin American countries, which have borne the brunt of the COVID-19 pandemic in terms of mortality and transmission.
The antigen-based rapid test can detect the presence of SARS-CoV-2 antigens in samples taken from the respiratory tract. The antigen is detected when the virus replicates actively, Adisasmito informed.
The antigen-based rapid test is best performed when someone has just been exposed to the virus, before antibodies are generated by the body to fight the virus.
Like the antibody rapid test, the antigen-based rapid test will likely not be accurate because the virus detected by the antigen may not be SARS-CoV-2, but another virus, such as influenza.
Related news: Indonesia's COVID-19 cases climb by 4,174 to 291,182
Related news: Vaccination to be prioritized for workers in 18-59 age bracket
Related news: Government disburses Rp304.6 trillion for handling COVID-19
Translator: Desca Lidya Natalia/Suharto
Editor: Sri Haryati
Copyright © ANTARA 2020