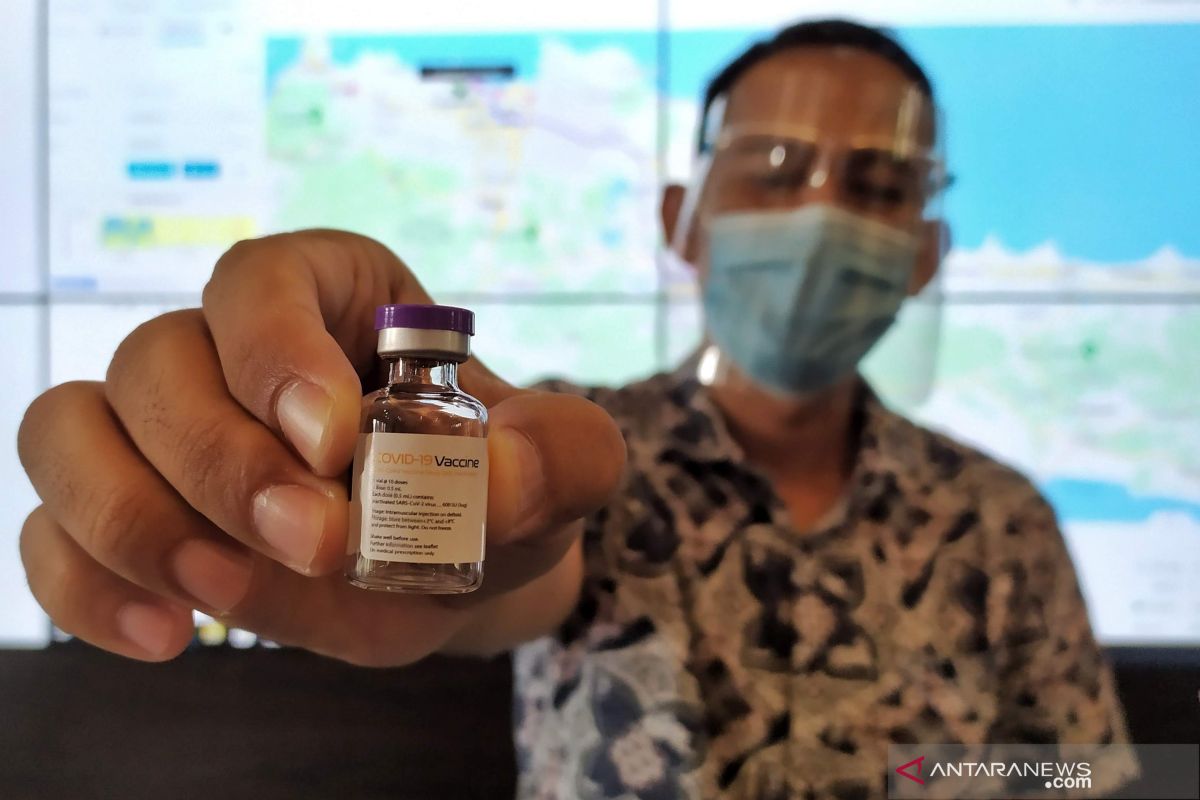
Once the bulk [shipment] arrives, the vaccine would be produced by Bio Farma, and people shall not feel worried as it is standardized.Bandung (ANTARA) - Indonesian pharmaceutical company Bio Farma has been authorized by the National Agency of Drug and Food Control (BPOM) to produce 100 million doses of China-based Sinovac’s COVID-19 vaccine.
"We have received a BPOM certification for 100 million doses, thus the production process is now aligned with BPOM standards, which is also internationally standardized," Minister of State-Owned Enterprises, Erick Thohir, said during his visit to Bio Farma in Bandung on Thursday.
Thus, Thohir added, Bio Farma is awaiting bulk vaccines to arrive in Indonesia, and the Chinese company is expected to make its shipment soon.
Related news: COVID-19 vaccine acceptance rests on halal status, safety
"Once the bulk [shipment] arrives, the vaccine would be produced by Bio Farma, and people shall not feel worried as it is standardized," Thohir remarked.
Bio Farma is now capable of producing up to 250 million doses of the vaccine, but for the production for the other 150 million doses, it will have to obtain another BPOM's certification, he said.
Thohir also emphasized that Bio Farma is currently still managing coordination with the Indonesian Ulema Council (MUI) on the vaccine's halal status.
"The halal issue would be on MUI; hence, we will not claim whether this vaccine is halal or not. [...] and we are talking to MUI regarding this matter," he said.
Related news: Government urged to guarantee halal status, safety of Sinovac vaccine
Translator: Bagus Ahmad Rizaldi, Suwanti
Editor: Rahmad Nasution
Copyright © ANTARA 2021