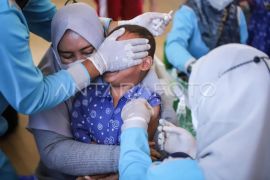
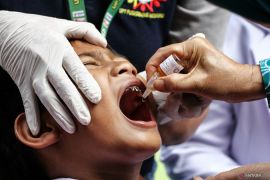
"The average rate of vaccination in the past week has increased slightly since child vaccinations began, with an average of 1,219,453 doses per day," Hartarto stated on Monday.
Vaccination for children in the age bracket of six to 11 years had increased the daily vaccination rate of over 300 thousand doses in the last four days. In total, 2,324,644 doses were administered to children aged six to 11 years.
Meanwhile, the government has targeted health workers, including supporting staff working at health facilities, elderly, and the beneficiaries of fund assistance, to receive the vaccine booster.
"This booster vaccine will be distributed by Biofarma and can be conducted in a homologous or heterologous way," Hartarto noted.
Related news: Indonesia's vaccination rate has surpassed WHO target: Deputy Minister
Meanwhile, the independent booster vaccination program targets other community groups mentioned above. The distribution of this independent booster vaccine will conducted by Biofarma and/or a pharmaceutical company that meets the standards of vaccine delivery/logistics and vaccination implementation and can be conducted in either a homologous or heterologous manner.
"The booster vaccine program still awaits the Indonesian Technical Advisory Group on Immunization's (ITAGI's) reports and recommendations on January 10, 2022," the minister noted.
Furthermore, Hartarto highlighted the latest developments pertaining to the Merah Putih Vaccine and State-Owned Enterprises (SOEs) vaccines. First, the Airlangga University (Unair)-PT Biotis vaccine is still awaiting approval for the Phase I clinical trials from the National Agency of Drug and Food Control (BPOM). Thereafter, the phase 2 and 3 clinical trials will be conducted in January-June 2022, while the Emergency Use Authorization (EUA) and Indonesian Ulema Council's (MUI's) fatwa are expected in the third quarter of 2022.
Related news: COVID-19 booster vaccine available to public from January 4: Minister
The Eijkman-Bio Farma's vaccine collaboration is in the pre-clinical testing stage and still awaiting the industrial Good Manufacturing Practice (CPOB) facilities. The EUA and MUI Fatwa are expected to be issued in the fourth quarter of 2022.
For the Bio Farma-Baylor College of Medicine's vaccine collaboration (SOEs vaccine), the clinical trial 1 has started on December 13, while the EUA and MUI Fatwa are expected to be completed in July 2022. The registration to obtain the World Health Organization's (WHO's) Emergency Use of Listing is expected to be completed on June 8, 2021, while the producer must be able to fulfill production capacity to reach 150 million doses during the June-December 2022 period.
Meanwhile, the development of SOEs vaccines and cooperation for domestic production, including GX-19 (Kalbe Farma & Genexine), is currently in the phase 3 of clinical trial to obtain the EUA. The EUA and MUI Fatwa are projected to be available in early 2022, with production capacity reaching 50 million doses in the June-December 2022 period.
Lastly, the Zifivax vaccine (JBio-Anhui Zhifei) has cleared the phase 3 clinical trials and readied a joint production facility for Biotis in Serang, Banten. The EUA for those in the age bracket of 18-59 years has been issued since October 7, 2021, with halal fatwa from MUI Number 35 of 2021. The producer is expected to produce 150 million vaccine doses in 2022.
Related news: Protocols for clinical trials of Merah Putih vaccine enter final stage
Translator: Kuntum K R, Resinta S
Editor: Suharto
Copyright © ANTARA 2021