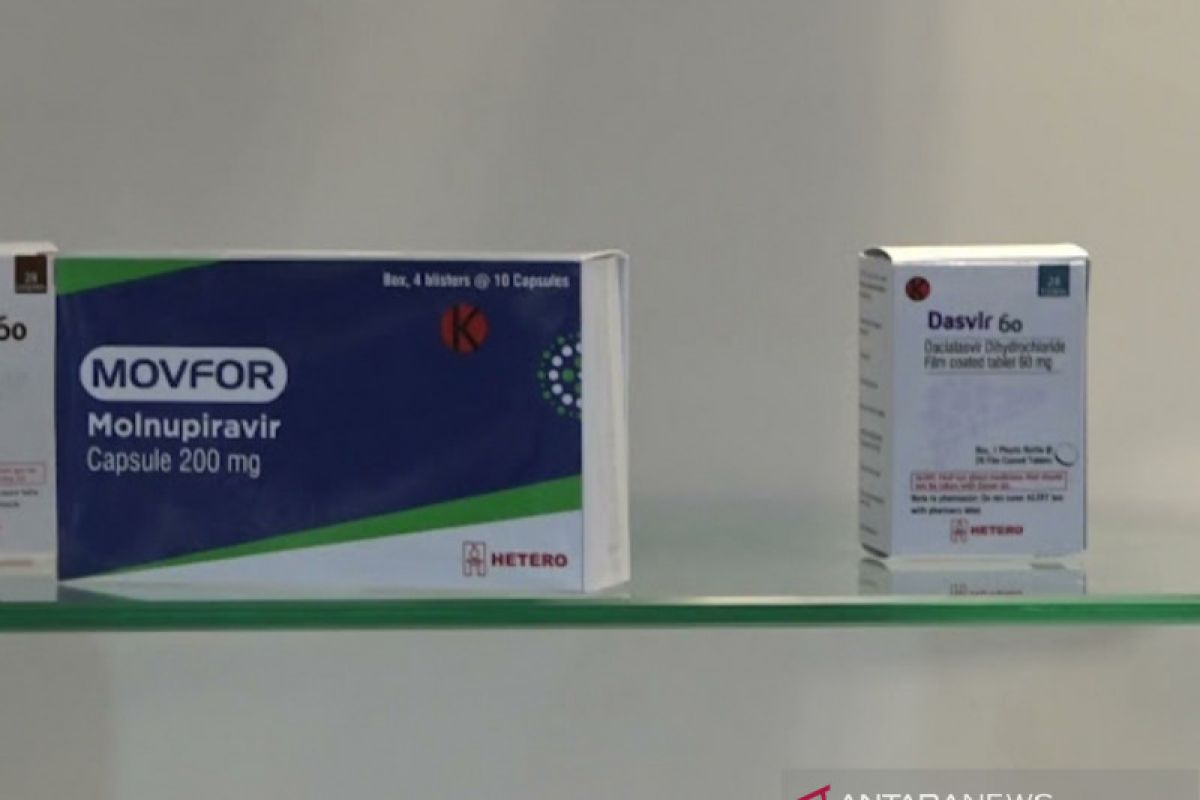
"After going through an evaluation of the clinical trial data together with the expert team of the National Committee for Drug Assessment and the clinical association for EUA approval, BPOM and the Ministry of Health continue to monitor the safety of the use of Molnupiravir in Indonesia," head of BPOM, Penny K Lukito, said in a press release received here on Friday.
The approved Molnupiravir medicine is in the form of 200 mg capsules registered by PT Amarox Pharma Global and manufactured by Hetero Labs Ltd., India, Lukito informed.
The drug is indicated to treat mild to moderate COVID-19 infection in patients aged 18 years and above who do not require oxygen administration and have an increased risk of developing severe COVID-19 infection, she said.
"The drug is given twice a day, as much as four capsules of 200 mg each, for five days," she noted.
Earlier, BPOM had issued EUA for several COVID-19 drugs, including antiviral drugs Favipiravir and Remdesivir and monoclonal antibody Regdanvimab.
The results of the evaluation have shown Molnupiravir is relatively safe and produces tolerable side-effects, Lukito said. The most commonly reported side-effects include nausea, headache, abdominal pain, and oropharyngeal pain, she added.
In addition, the results of non-clinical trials and clinical trials have shown that Molnupiravir does not cause liver function disorders, she noted. However, Molnupiravir should not be prescribed to pregnant women and women of productive age who are using contraceptives, she added.
Regarding the efficacy aspect, the results of the Phase Three clinical trial have shown that Molnupiravir could reduce the risk of hospitalization by 30 percent in mild to moderate COVID-19 patients and 24.9 percent in mild COVID-19 patients, she informed.
Related news: Indonesia aims to start Molnupiravir production in April
To support of the availability of COVID-19 drugs in Indonesia, PT Amarox Pharma Global is preparing for local production of Molnupiravir capsules through transfer technology at the Amarox Cikarang production facility in Bekasi District, West Java, she informed.
“A letter of approval for the use of non-betalactam capsule production facilities was issued on January 3, 2022, and after the requirements for Good Manufacturing Practices of Drugs (GMP) can be met by the industry, the local production is planned to be ready in early March 2022. This enhances our joint efforts to support the independence of the domestic drug industry," she said.
In addition, BPOM is also supervising the production and distribution chain of drugs so that the safety, efficacy, and quality of drugs can be maintained to prevent the use of illegal drugs, she added.
Related news: Omicron tally reaches 152: Health Ministry
Translator: Andi Firdaus, Resinta S
Editor: Rahmad Nasution
Copyright © ANTARA 2022