The opportunities to cooperate with national private companies are now open as wide as possibleJakarta (ANTARA) - The Indonesian government is open to cooperating with national private companies for producing and distributing the Red and White COVID-19 vaccine, which is currently under development, according to the COVID-19 Response Task Force.
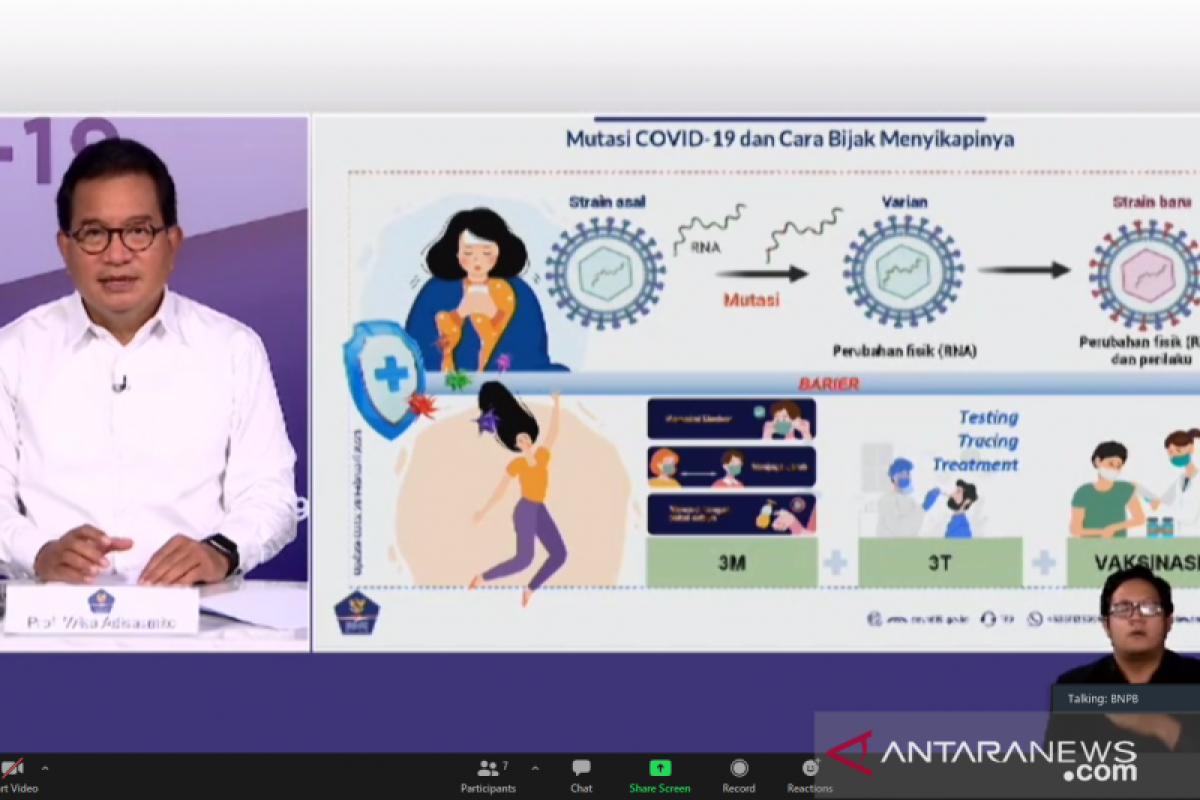
"The opportunities to cooperate with national private companies are now open as wide as possible, under the coordination of the government, to allow the downstream industry to increase production capacity, facilitate pre-clinical processes and clinical trials and expand the targeted market," coordinator of the expert team and government spokesperson for COVID-19 handling, Wiku Adisasmito, said in Jakarta on Thursday.
He said the Eijkman Institute for Molecular Biology, in collaboration with non-ministerial government institutions and several state-owned institutes of higher learning, is in the process of conducting research for the development of the Red and White vaccine, named after the Indonesian flag.
The involvement of private companies will accelerate the attainment of vaccine production target, Adisasmito said.
"(The involvement of private firms) will not only ensure the fulfillment of vaccine needs but also serve as potential for Indonesia in the future. It will play an active role in attaining global health security for countries that have access to COVID-19 vaccines," he added.
Meanwhile, Minister of Research and Technology, Bambang PS Brodjonegoro, said the activities related to the research and development of the Red and White vaccine have reached 100 percent on the laboratory scale.
“According to the plan, in March, 2021, maybe late March, we will begin to hand over vaccine seeds to state-owned pharmaceutical firm Bio Farma so the laboratory phase has been 100 percent completed," he informed.
Bambang said the Eijkman Institute has set itself the target of submitting vaccine seeds, developed using a recombinant protein subunit platform, to PT Bio Farma in March this year.
After receiving the vaccine seeds, Bio Farma must conduct the optimization and purification process to clean the vaccine seeds and later carry out clinical trials, he added.
After a series of clinical trials have been completed and the vaccine secures emergency-use authorization from the Drug and Food Control Agency (BPOM), mass production will begin, he informed.
Related news: COVID-19: Vaccine still effective against UK variant, assures govt
Related news: Military, Police urged to assist in expediting vaccination: Minister
Related news: Over two million Indonesians vaccinated so far: task force
Translator: Asep Firmansyah/Suharto
Editor: Sri Haryati
Copyright © ANTARA 2021