We encourage domestic and foreign manufacturers to fulfill the supply for COVID-19 drugs.Jakarta (ANTARA) - The Health Ministry on Wednesday afternoon flagged a shortage in stocks of three types of therapeutic drugs used in the treatment of COVID-19 in Kimia Farma's pharmacy network.
According to the Farma Plus website, 3,114 Kimia Farma stores across Indonesia reported insufficient stocks of Immunoglobulin, Remdesivir, and Tocilizumab.
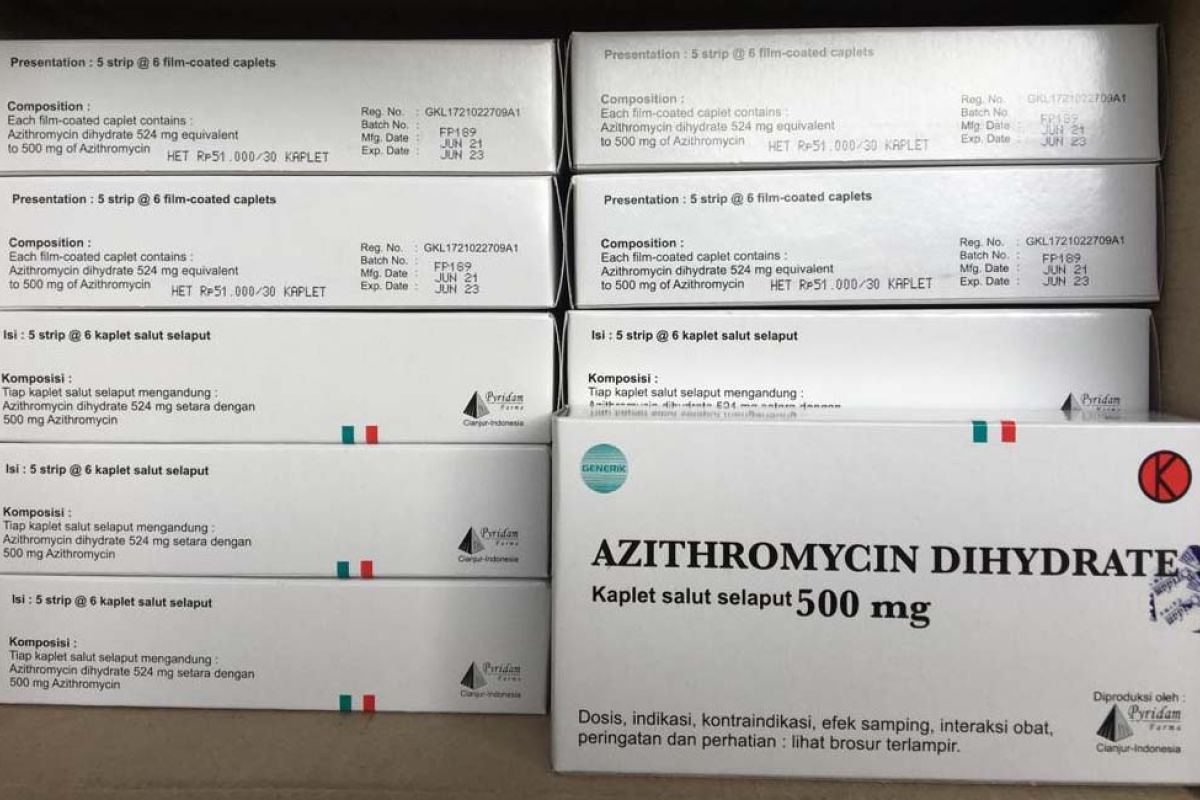
Meanwhile, 672 thousand drugs remained available in the network, comprising 134 thousand Azithromycin units; 349.9 thousand Favipiravir units; 178.7 thousand Ivermectin units; and 9,971 Oseltamivir units.
During a virtual meeting with Commission IX of the Indonesian House of Representatives (DPR) on Tuesday (July 13, 2021), Indonesian Health Minister Budi Gunadi Sadikin had said that the government will allocate additional supplies of COVID-19 therapies gradually until the end of July, 2021.
The additional drugs comprise 11.2 million Azithromycin units out of the estimated 1.5 million units needed by the country; 6.2 million Ivermectin units against the estimated demand for 1.7 million units; and, 5.7 million Oseltamivir units out of the estimated demand for 4.2 million units.
They also included 1.4 million units of Remdesivir as against the 1.6 million units needed by the country; 8 million units of Favipiravir as against the demand for 12 million units; 73,660 units of IV Immunogobulin against the demand for 1.2 million units; and, 3,800 units of Tocilizumab (Actemra) against the total need for 60,162 units.
"We encourage domestic and foreign manufacturers to fulfill the supply for COVID-19 drugs. For Tocilizumab and IV Immunogobulin, we urge global producers (Actemra-Roche) to prioritize the supply of their products to Indonesia. Meanwhile, additional supply alternatives will be acquired from other manufacturers, such as China, through the Special Access Scheme (SAS) and donations," Sadikin said.
Regarding Remdesivir supplies, the government has been trying to raise the quota of imported products from India, Bangladesh, Egypt, and China, he informed. Meanwhile, Favipiravir would be obtained through accelerating and increasing domestic production, he said.
Related news: Action will be taken against those disrupting COVID drug supplies
Related news: Kimia Farma producing, distributing three therapeutic COVID drugs
"We also encourage even distribution of drugs among the regions to prevent severe shortages," he remarked.
Speaking over the telephone from Jakarta on Wednesday, director of communicable disease prevention and control at the Health Ministry, Siti Nadia Tarmizi, confirmed that the government is continuing to coordinate with pharmaceutical industries and distributors to monitor the availability of medicines in Indonesia.
"The Health Ministry is also coordinating closely with the pharmaceutical industry and distribution networks to monitor the availability of drugs needed for handling COVID-19," she said.
The Health Ministry has issued a Minister of Health Decree No.HK.07.07/Menkes/4826/2021 to regulate the Highest Retail Prices (HET) for drugs amid the COVID-19 pandemic.
Related news: Minister assures COVID-19 drug stocks adequate
Related news: Govt to distribute 300 thousand packages of COVID-19 therapy drugs
Translator: Andi Firdaus, Uyu Septiyati Li
Editor: Fardah Assegaf
Copyright © ANTARA 2021