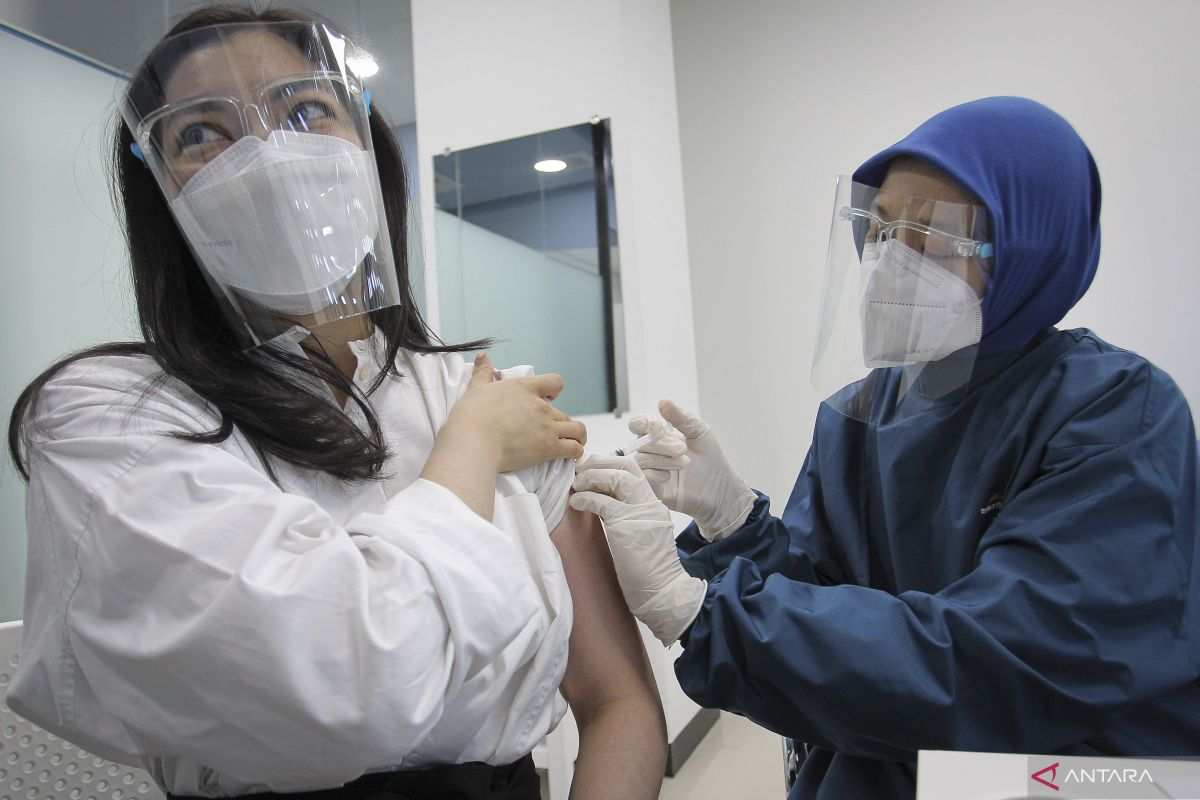
"Alhamdulillah (thank God), today we will carry out the phase three clinical trial," Director of Bio Farma Honesti Basyir said at the kick-off event for the phase three clinical trial of the SOEs vaccine, which was monitored from here on Thursday.
The third phase clinical trial of the SOEs vaccine involves as many as 4,050 subjects aged 18 to 70 years.
Basyir affirmed that if the clinical trial goes well and receives an emergency use authorization, Bio Farma will start production of the vaccine in July 2022.
"We have prepared a fairly large production capacity, where for this SOEs vaccine we have prepared a capacity of 120 million doses per year," he said.
Bio Farma is planning to use the vaccine for booster vaccinations and children's vaccinations.
Meanwhile, the Head of the National Agency for Drug and Food Control (BPOM), Penny Lukito, said the SOEs vaccine was the first vaccine whose entire development was carried out in Indonesia.
The research for the SOEs vaccine was obtained from abroad, but its development into a vaccine that meets various commercial product standards has been carried out in Indonesia, starting from the pre-clinical phase, first phase clinical trial, second phase clinical trial, to final phase clinical trial.
According to Lukito, the development of the SOEs vaccine is a giant step for Indonesia, in reaching drug and vaccine independence.
"Thanks to partners from industries who, together with the BPOM, have followed international standard rules. We want the vaccines that are developed in Indonesia to become products that can compete. Hopefully, we can finish this on time," the BPOM head noted.
Related news: Bio Farma explores Merah Putih vaccine exports in endemic phase
Related news: Merah Putih Vaccine heading for phase 3 clinical trial
Translator: Sugiharto Purnama, Raka Adji
Editor: Rahmad Nasution
Copyright © ANTARA 2022