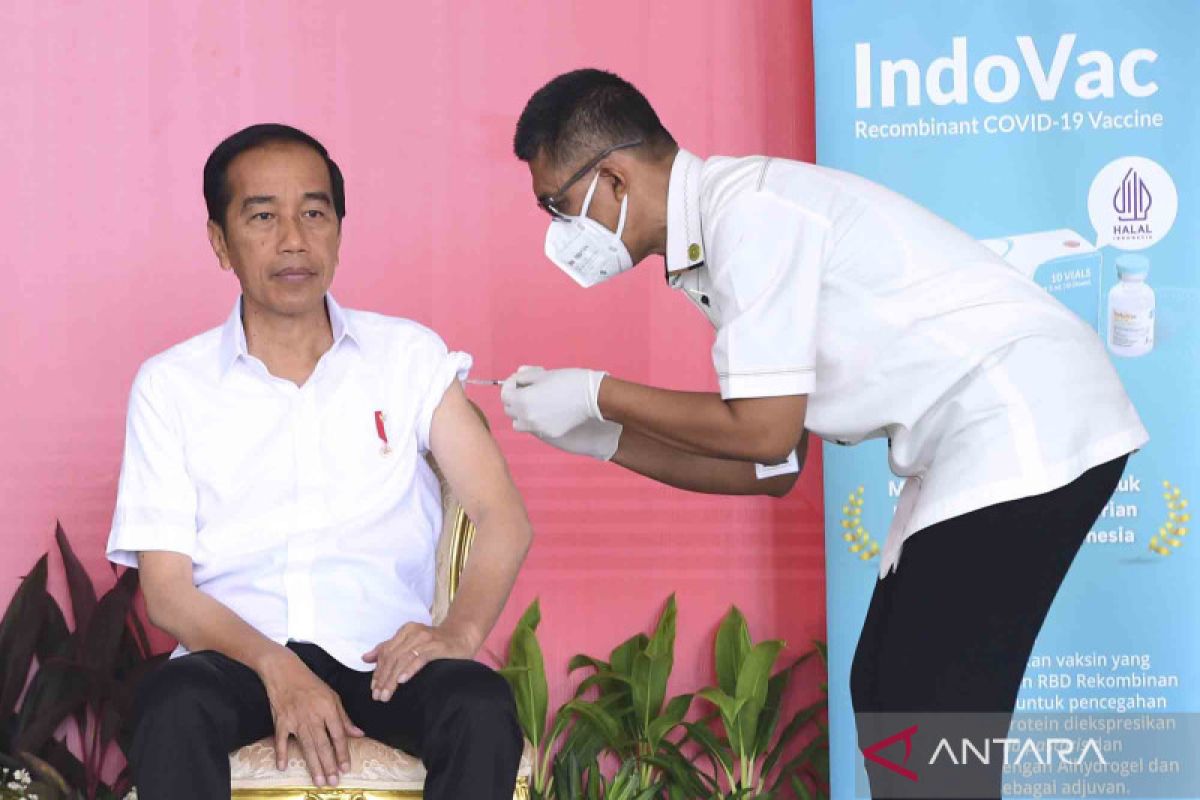
We will prepare the IndoVac vaccine for booster vaccination of the elderly group.Jakarta (ANTARA) - Bio Farma is ready to produce five million doses of the IndoVac vaccine by 2022-end to support the second booster vaccinations for the elderly, president director of the state-owned pharmaceutical company, Honesti Basyir, has informed.
"We will prepare the IndoVac vaccine for booster vaccination of the elderly group. The production process has been underway since the issuance of the Emergency Use Authorization (EUA) for primary doses at the end of September 2022," Basyir said in a press statement received here on Friday.
In its circular letter Number HK.02.02/C/5565/2022 concerning the second booster dose of the COVID-19 vaccination for the elderly group, issued on November 22, 2022, the Health Ministry said that the elderly can be provided IndoVac as the second booster.
Related news: Indonesia entering fourth COVID wave, IDI predicts
Basyir said that the use of IndoVac could increase the coverage of booster vaccinations, which has only reached 36 percent.
"We hope that with the issuance of the ministry's circular letter, the IndoVac vaccine can help achieve the target of COVID-19 booster vaccination in Indonesia, especially for the elderly," he added.
The booster vaccine is necessary, especially for the elderly who are vulnerable to the virus, to provide additional protection and most importantly, to reduce the severity of infection and death from COVID-19.
The second booster vaccination for the elderly could be provided at healthcare facilities or COVID-19 vaccination facilities.
The IndoVac vaccine, which was launched by President Joko Widodo on October 13, received EUA for the primary dose at the end of September.
Not long after, it obtained a halal certificate from the Indonesian Ulema Council (MUI).
The EUA for the use of IndoVac as a booster vaccination was issued by the Food and Drug Supervisory Agency (BPOM) in early November.
Related news: IndoVac passes clinical trial for COVID booster: Bio Farma
As a booster dose, the vaccine would be provided at intervals of at least six months after the administration of the primary dose. It can be used for those who have received the Sinovac vaccine as the primary dose.
The IndoVac vaccine will be provided at the full dosage or 0.5 ml.
According to Basyir, booster vaccination is important as on average, people would have received their second dose more than six months ago, thus, there would be potential for a decline in immunity against the COVID-19 virus.
The COVID-19 booster vaccination aims to increase the level of antibodies against the virus in people who have received the complete primary dose of the COVID-19 vaccine.
Related news: Eye on endemic status, IDI asks people to get COVID booster
Bio Farma has conducted clinical trials of IndoVac for children aged 7–17 years in collaboration with Andalas University (UNAND), the University of Indonesia (UI), and Gadjah Mada University (UGM).
The EUA is targeted to be released in early December 2022.
The first administration of the second booster was observed by President Widodo, Minister of State Secretary, Pratikno, Health Minister Budi Gunadi Sadikin, State-Owned Enterprises Minister Erick Thohir, Public Works and Public Housing Minister Basuki Hadimuljono, and Basyir.
Indonesia has so far administered 205 million first doses of the COVID-19 vaccine, 172 million second doses, 66 million doses first boosters, and 730 thousand second boosters.
Related news: Local COVID vaccines to help meet booster vaccine needs: Ministry
Related news: Indonesians advised vigilance as COVID shadow looms again
Translator: Ida Nurcahyani, Sri Haryati
Editor: Fardah Assegaf
Copyright © ANTARA 2022