IndoVac is a locally-made COVID-19 vaccine produced by Bio Farma.
"If the government wants a COVID-19 vaccination program for children (by using IndoVac), we can finish the clinical trials in three months," Bio Farma President Director Honesti Basyir said here on Tuesday.
COVID-19 vaccinations for children aged 6–11 were officially launched by the government on December 14, 2021.
However, currently, the stock of the Sinovac vaccine, which is used in children's vaccination, is facing a shortage since the government decided to stop vaccine imports as of October 2022 and switch to domestically produced vaccines.
Even though the vaccination, which is targeting 26.5 million children, has been running for almost a year, the vaccine stocks that Indonesia has are not much, Basyir noted.
He said that advanced clinical trials of the IndoVac vaccine carried out by Bio Farma are currently targeting the use of the vaccine as a booster vaccination for adolescents aged 12–17.
The clinical trials need to continue to be carried out in stages to ensure the produced vaccine is safe and has good efficacy for each target age group, he added.
"Currently, there is no vaccine for children aged 6–11 because the program targets high-risk groups first," he said.
The World Health Organization (WHO) has not set children aged 6–11 as a high-priority group, he added.
He informed that clinical trials of the IndoVac vaccine for adolescents are targeted to be completed by the end of 2022.
As part of efforts to boost community immunity against COVID-19, the Indonesian government rolled out a nationwide vaccination program on January 13, 2021, targeting as many as 234,666,020 citizens.
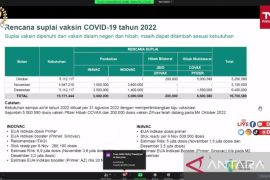
According to data provided by the COVID-19 Handling Task Force, as of December 12, 2022, as many as 203,833,159 Indonesians have received the first vaccine dose, 174,449,231 have been administered the second dose, 67,522,970 have taken the third dose or first booster, and 1,018,280 have received the fourth dose or second booster.
Related news: Polio vaccine side effects milder than disease: IDAI
Related news: Committee collects COVID-19 vaccine data for children under six
Translator: Andi Firdaus, Raka Adji
Editor: Rahmad Nasution
Copyright © ANTARA 2022