BPOM head Taruna Ikrar stated on Thursday that this TB vaccine trial offers significant benefits for Indonesia, including renewed strategies for TB management. He noted that Indonesia has the second highest number of TB sufferers after India.
He remarked that currently, TB treatment involves several drugs: isoniazid, rifampicin, and ethambutol.
"The combination of these three drugs, due to their long-term use, causes some kind of resistance that is hard to cure," he stated.
Meanwhile, Ikrar pointed out that the existing Bacillus Calmette-Guérin (BCG) vaccine is considered less effective in combating the disease.
"Therefore, the discovery of this new technology, with these new results, is expected to be beneficial for our people," he affirmed.
In addition, participation in the M72 tuberculosis vaccine clinical trial could reduce imports of raw materials for drugs, as participating countries may obtain intellectual property rights to produce the vaccine domestically, he stated.
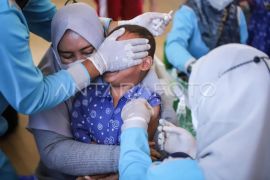
He reported that around two thousand Indonesians are participating in this phase 3 clinical trial, with a total of 20 thousand global participants.
"The two thousand samples will be double-blinded, meaning they may receive the vaccine or only the vehicle," Ikrar explained.
"The production process after the test results will involve Biofarma. Biofarma and BPOM will control its good manufacturing practices," he stated.
On that occasion, Biofarma President Director Shadiq Akasya welcomed the phase 3 clinical trial of the TB vaccine and expressed hope for a successful outcome without setbacks.
He also expressed optimism for continued support from the Gates Foundation and BPOM to enable local vaccine production.
Meanwhile, Senior CMC Advisor Vaccine Development Gates Foundation Rasayam Prasad stated that vaccine development aims not only to save lives but also to improve vaccine accessibility.
"In the future, we look forward to developing another vaccine, not only for Indonesia but establishing Indonesia as a hub for supplying vaccines globally, for diseases such as tuberculosis, measles, rubella, pneumonia, rotavirus, and of course, polio," Prasad added.
Related news: Indonesia's BPOM ensures safety, benefits of TB vaccine trial
Related news: RI Govt assures of safety of M72 TB vaccine trial in Indonesia
Related news: Minister ensures phase 3 TB vaccine study safety
Translator: Mecca Yumna, Resinta Sulistiyandari
Editor: Azis Kurmala
Copyright © ANTARA 2025