One key advantage, he said on Wednesday, is that Indonesians will be among the first to receive the TB vaccine.
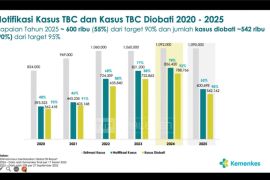
He added that the vaccine is crucial as Indonesia ranks second globally in TB cases, with more than 1 million infections and approximately 125,000 deaths recorded in 2023.
Ikrar emphasized that vaccination is one of the most effective strategies to reduce TB prevalence in the country, where the disease manifests in multiple forms.
“That is why I believe this will be highly beneficial for the public. There are many advantages,” he noted.
Related news: Indonesia strategic force in ending TB globally, says Bill Gates
He explained that TB vaccination could also help prevent the rise of drug-resistant TB cases, which are currently affecting several Indonesians.
Ikrar assured the public that the vaccine to be trialed is safe, having undergone preclinical testing, toxicity studies, and safety trials conducted according to international standards.
“Based on scientific evaluations, we have authorized the clinical trials of the TB vaccine in Indonesia,” he said. “There is no issue here. The TB vaccine will bring many benefits.”
Earlier, Minister of Health Budi Gunadi Sadikin confirmed the safety of the M72 tuberculosis vaccine following the successful completion of its first phase of clinical trials.
He expressed hope that the vaccine could be launched globally before 2029.
Related news: RI Govt assures of safety of M72 TB vaccine trial in Indonesia
Related news: Bill Gates, Prabowo discuss TB vaccine trials in Indonesia
Translator: Sean, Kenzu
Editor: Anton Santoso
Copyright © ANTARA 2025