Furthermore, no reports were received of serious undesirable incidents from volunteers that were suspected of being related to vaccines or vaccination activities.
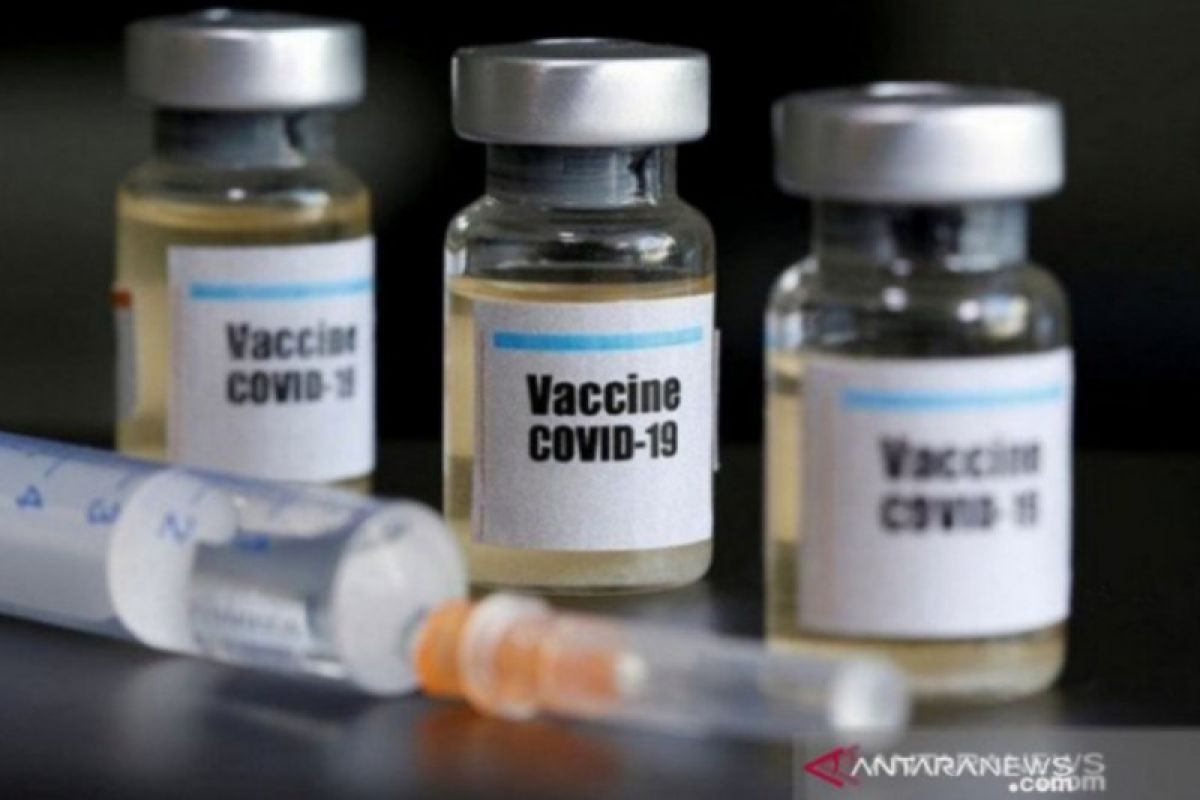
Spokesperson for the Clinical Trial Team for Phase Three of the COVID-19 Vaccine Dr. Rodman Tarigan stated in Bandung on Wednesday that the COVID-19 vaccine, the result of collaboration between Bio Farma and Sinovac, had currently entered the monitoring period.
Data as of November 6, 2020, showed 1,620 volunteers had received the first injection, 1,603 volunteers had been administered the second injection, and 1,335 volunteers had entered the monitoring stage, for immunogenicity, efficacy, and safety.
SAE is one of the adverse events following KIPI experienced by drug or vaccine recipients, regardless of the relationship with the drug or vaccine, while a non-serious adverse event or mild adverse event constituted medical events after vaccination and did not pose a potential risk to the health of recipients, such as fever and swelling and redness at the injection site.
Every volunteer, who received the first and second injections, until the clinical trial is complete, will be supervised and monitored by the clinical trial team, so that whatever incident befalls the volunteer must be monitored.
Novilia, one of the members of the Bio Farma Pharmacovigilance Expert Team, stated that the SAE experienced by an individual can arise both for vaccines that have been marketed and vaccines currently in the clinical trial stage, such as the COVID-19 vaccine.
He expounded that for products in the phase of clinical trials, SAE will be reported to the Ethics Committee, Food and Drug Monitoring Agency (BPOM) and DSMB (Data Safety Monitoring Board), while marketed products will be investigated, as well as analysis by independent institutions, such as KOMNAS KIPI, and reported to the BPOM to ascertain whether the main cause of this event is directly related to the vaccine or other factors as coincidence.
For SAE incidents currently occurring in Brazil, he highlighted the need for further investigations to determine whether SAE was related to vaccines or co-incident.
In this SAE investigation, the local Drug Supervisory Agency will certainly be involved. Pause or Postponement of the implementation of clinical trials of drugs or vaccines is a standard procedure and is commonly used to conduct prior investigations over serious adverse events following immunization found in research.
Regarding the SAE COVID-19 Sinovac Vaccine case in Brazil, an official statement was issued by Sinovac at http://www.sinovac.com/?optionid=754&auto_id=914 wherein Sinovac has communicated with the Butantan Institute and stated that the SAE incident was not found to have any relation to the vaccine.
Vaccines proffer vast benefits in terms of breaking the chain of transmission of infectious diseases. Vaccines are a means of preventing infectious diseases that are not only administered to infants but also to adults. Vaccines not only provide individual immunity but also create mass immunity, also known as herd immunity.
Vaccines can also prevent diseases that can cause death or disability. Data from the World Health Organization (WHO) shows that nearly 10 million deaths can be prevented through vaccination.
Related news: Govt to inject Rp2 trillion into Bio Farma for vaccine procurement
Related news: Airlangga University developing two COVID-19 vaccines
EDITED BY INE
Translator: Ajat Sudrajat, Azis Kurmala
Editor: Fardah Assegaf
Copyright © ANTARA 2020