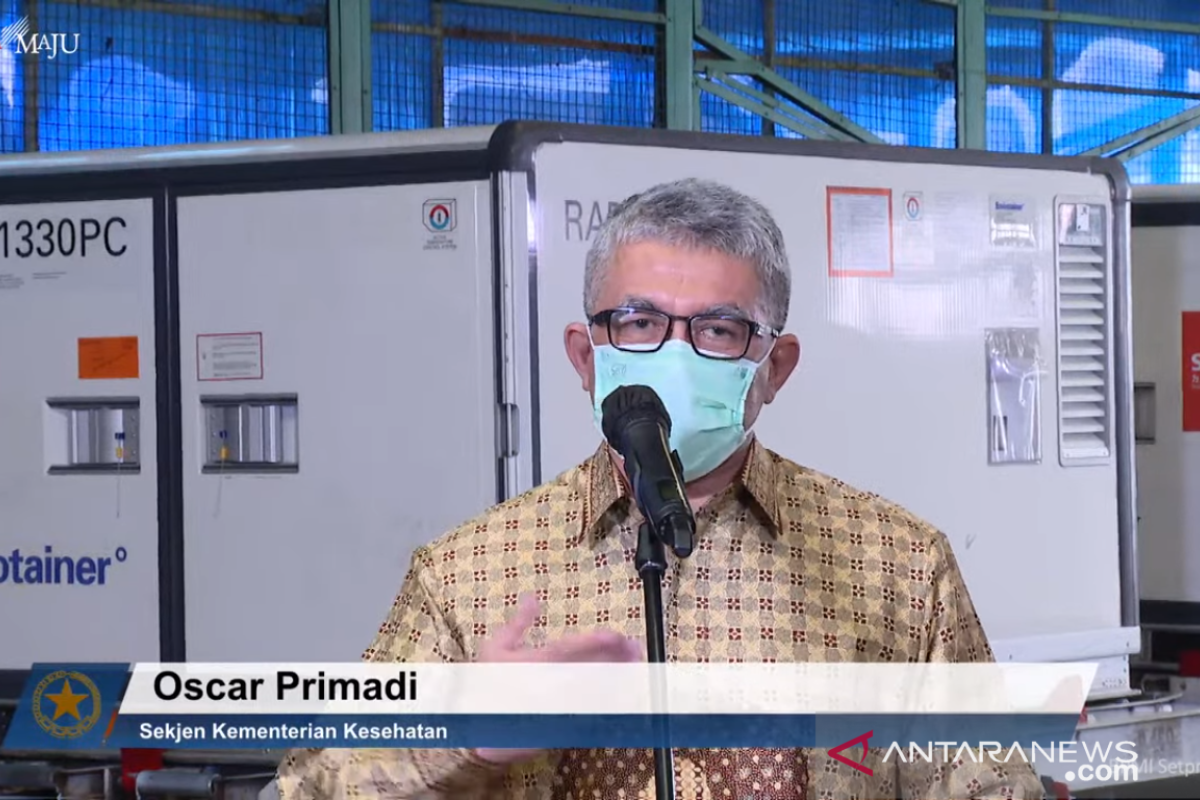
"The vaccines provided or prepared by the Health Ministry have been tested from various aspects of safety, efficacy and quality," said Secretary General of the Health Ministry Oscar Primadi in a virtual press conference held after the arrival of another 10 million doses of Sinovac bulk vaccine at Soekarno-Hatta Airport in Tangerang, Banten province, on Sunday.
The Food and Drug Control Agency issued an emergency use authorization (EUA) to the vaccines that have met the aspects. After that, the vaccines could be used for the COVID-19 vaccination program in order to give benefit to recipients ,
On Sunday Indonesia received 10 million doses of Sinovac bulk vaccines to produce vaccines to meet the target of vaccinating 181.5 million Indonesians, so that the public will gain herd immunity against COVID-19.
As of June 20, 2021, Indonesia has received 104,728,400 doses of COVID-19 vaccine, comprising 94,500,000 doses of Sinovac vaccine, 8,228,000 doses of AstraZeneca vaccine and 2 million doses of Sinopharm vaccine.
Primadi said the procurement of the vaccine was carried out through various efforts, including bilateral cooperation and the development of a domestic vaccine called Merah Putih (Red and White) vaccine.
He said the arrival of the 10 million doses of Sinovac bulk vaccine would encourage the smooth implementation of the COVID-19 vaccination program.l.
He ensured that government will make constant efforts to accelerate and strengthen the vaccination program.
"But, once again we urge the community to adhere to health protocols, avoid crowds, maintain social distance, and wear masks," said the secretary general.
Related news: Indonesia gets another 10 million doses of Sinovac bulk vaccines
Related news: Indonesia has safe supply sources for procurement of COVID-19 vaccines
Translator: Prisca Triferna Violleta, Katr
Editor: Suharto
Copyright © ANTARA 2021