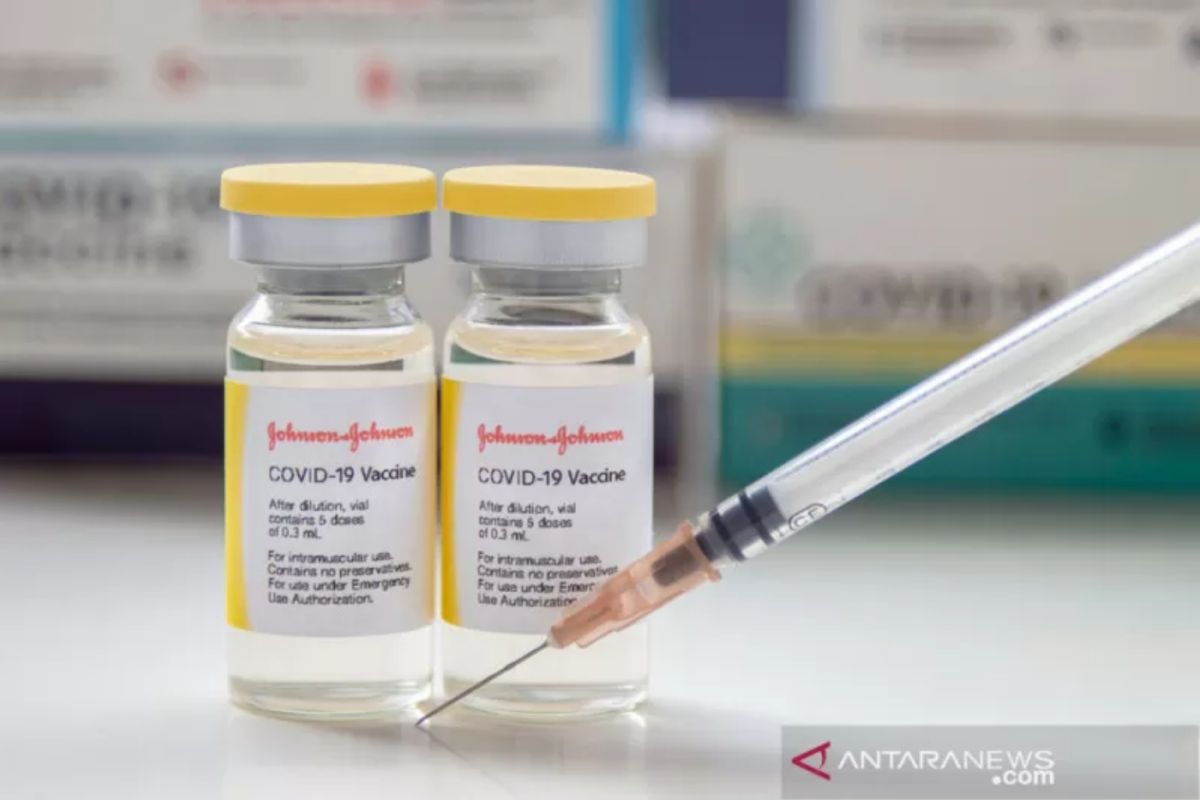
"On September 11, Indonesia received the Johnson & Johnson vaccine, which has been granted 'Emergency Use Listing' (EUL) by the WHO and an 'Emergency Use Authorization' (EUA) issued by the National Agency of Drug and Food Control (BPOM)," Prof. Aditama noted in a written statement received here Wednesday morning.
The Johnson & Johnson vaccine was projected to be given as a single dose and was considered to offer more advantages, as it was more practical with only one injection.
However, at a meeting attended by several international vaccine experts on September 21, Professor Aditama remarked that several research results were discussed regarding the use of two doses of the Johnson & Johnson's vaccine.
At the same time, Johnson & Johnson vaccine company revealed that two doses of its vaccine could offer up to 94-percent protection from the virus.
"This figure is equal to the level of protection offered by Moderna or Pfizer vaccines, which are indeed given in two doses. The manufacturer also noted that the additional second dose of the Johnson & Johnson vaccine will also boost immunity and protect against very severe infections," he affirmed.
According to Aditama, Johnson & Johnson's representative had stated that one dose of the vaccine can produce a strong immune response and also preserve immune memory for a long time.
Related news: J&J vaccine to be distributed to 7 agglomeration areas
"If the recipient receives the second dose, the power of protection against COVID-19 will increase again," he noted.
Johnson & Johnson has submitted a plan to administer two doses of vaccination since August 2021. They will hold discussions with relevant health officials about a possible strategy to administer the second dose after eight months or more after the first dose, Aditama explained.
The former Director of Southeast Asia WHO stated that this development was not widely known in Indonesia since the Johnson & Johnson vaccine is commonly identified for one dose of injection.
"This new development will certainly be a further study by the government in determining Johnson & Johnson's vaccination policy, which has only arrived in our country in a few days," he noted.
Aditama affirmed that the knowledge about COVID-19 was indeed very dynamic, with the possibility of changing in accordance with the results of the latest research.
Related news: 3rd batch of US-donated 1.1-million Pfizer doses arrive in Indonesia
Translator: Andi Firdaus, Resinta S
Editor: Rahmad Nasution
Copyright © ANTARA 2021