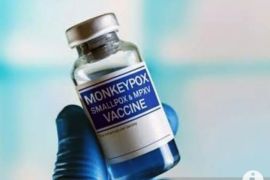
"Monkeypox vaccines that are currently available internationally are Jynneos and Smallpox vaccines, and they can be used, but through expanded access," Lukito noted while attending the Commission IX Opinion Hearing Meeting (RDP) streamed on YouTube, Tuesday.
Jynneos vaccine for adults has received the emergency use authorization (EUA) by the Food and Drug Administration (US-FDA) in 2019 and the European Medicines Agency (EMA) on July 22, 2022.
In addition, it received the EUA from Australia's Therapeutic Goods Administration (TGA) on August 5, 2022, and Health Canada on November 12, 2020.
Meanwhile, smallpox vaccine comprises Dryvax as the first generation and ACAM1000 and ACAM2000 as the second generation.
In the third generation, smallpox vaccine consists of the Modified vaccinia Ankara produced by Bavarian Nordic (MVA-BN), with the trademark Imvanex in Europe, Imvamune in Canada, and Jynneos in the United States.
Japan also produces the third generation smallpox vaccine, with the name LC16m8, produced by Kaketsuken.
Related news: BPOM readies three strategies to obtain monkeypox vaccines, drugs
While smallpox vaccine is 85-percent effective against monkeypox, access can only be obtained through expanded access, Lukito remarked.
To this day, monkeypox and smallpox vaccines have not been registered in Indonesia. Pox vaccine that is available in Indonesia is the varicella type for chickenpox, she noted.
"The BPOM can grant access to various alternatives to expedite access if the two vaccines will be procured in Indonesia," she remarked.
Medicine for monkeypox patients that is currently available globally is named Tecovirimat that is developed and utilized for smallpox medication, she noted.
The medicine has been approved by EMA for monkeypox according to studies on animals and humans.
However, US-FDA has not yet given Tecovirimat approval for monkeypox even though it is used in the United States through expanded access.
Related news: Indonesia has ordered 2,000 monkeypox vaccine doses: Health Minister
Related news: High-risk groups prioritized to receive monkeypox vaccine: IDI
Translator: Andi Firdaus, Fadhli Ruhman
Editor: Suharto
Copyright © ANTARA 2022