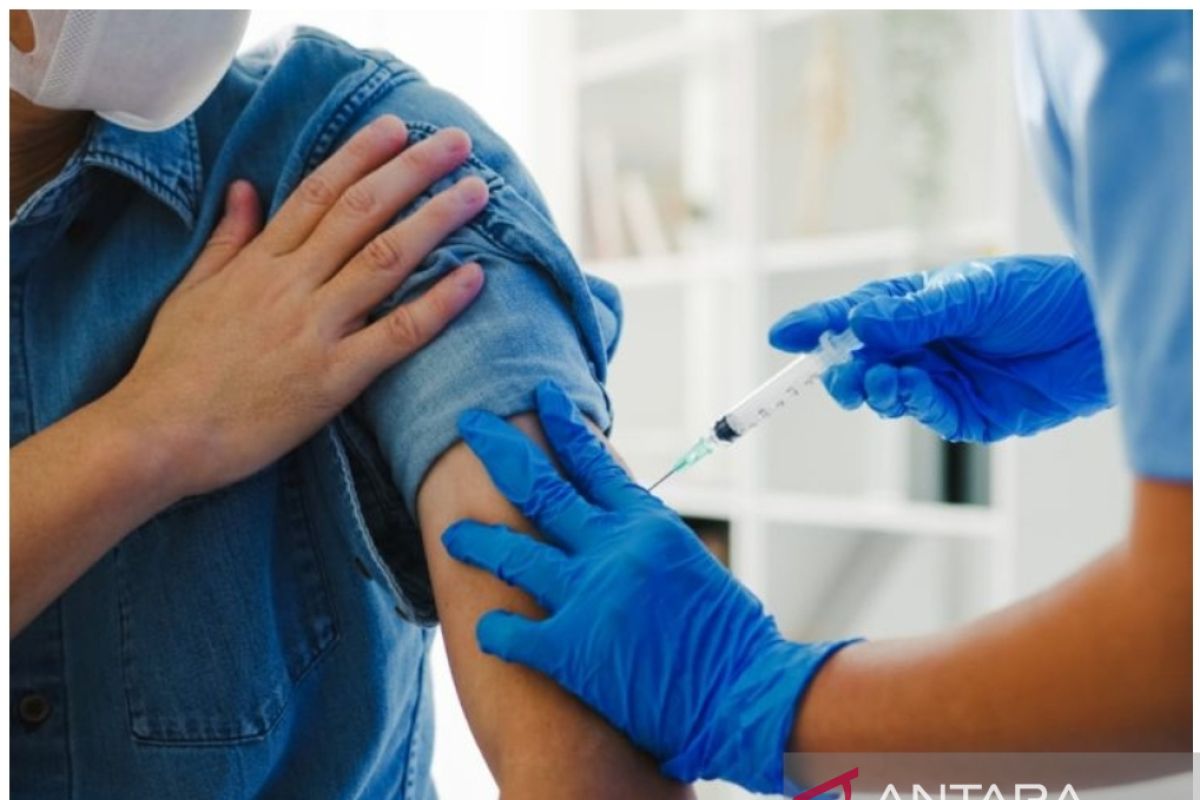
"Indeed, there is limited stock of the COVID-19 booster vaccine in Indonesia because we are still awaiting the arrival of the next batch," the ministry's Head of Health Communication and Public Service Bureau, Siti Nadia Tarmizi, stated in Jakarta, Thursday.
According to data from the COVID-19 Vaccination Dashboard of the Indonesian Ministry of Health as of Thursday, the people in Bener Meriah District, West Nias, Sawah Lunto, Pringsewu, Belitung, and Ciamis were facing a shortage of booster vaccine stocks.
The same situation was also experienced in the districts of Barito Kuala, Hulu Sungai Tengah, Hulu Sungai Utara, Tanah Bumbu, Raja Ampat, Paniai, Mimika, Waropen, and Dogiyai.
According to the dashboard information, 22 districts and cities had booster vaccine stocks that would last for the next seven to 10 days; 15 districts and cities, with stocks for the next 10 to 14 days; and 61 districts and cities, with less than a week of booster vaccine stock.
The ministry is currently relocating supplies of booster vaccines from areas that still have surplus vaccine stocks to other areas where supplies are running low or empty.
"For now, we are relocating from areas that still have a lot of stock to other areas that are still lacking," Tarmizi remarked.
One step being currently taken is the relocation of booster vaccines from North Maluku that currently has 2,127,436 doses of vaccine in stock.
Tarmizi stated that as many as five million doses of COVID-19 vaccine supplies in the country still meet the needs of the community. A total of 2.5 million doses are stored in facilities owned by the central government, while the rest are scattered across regional storage facilities.
Meanwhile, the ministry's spokesperson, Mohammad Syahril, stated that the vaccine relocation mechanism was conducted by considering the rate of vaccination in the area based on community requests.
"There are areas that run out of vaccines quickly because of the large number of people, who want to be vaccinated, but there are also those who do not want to receive vaccination," he remarked.
Currently, the ministry is managing the process of mobilizing vaccines to areas in need according to requests from the local health service.
The ministry is also pushing efforts to procure booster vaccines through a priority scale for purchasing domestically produced vaccines this year.
Under the mechanism, coordination was forged with state-owned pharmaceutical holding, PT Bio Farma, as producer of the IndoVac Vaccine, for procurement plans and industrial production readiness.
IndoVac is a COVID-19 vaccine containing the recombinant Receptor-Binding Domain (RBD) protein S of the SARS-Cov-2 virus, with a recombinant protein subunit platform developed by PT Bio Farma in collaboration with Baylor College of Medicine, the United States.
The National Agency of Drug and Food Control (BPOM) has issued an Emergency Use Authorization (EUA) for the IndoVac Vaccine on September 24, 2022.
Bio Farma produced around 20 million doses of the COVID-19 vaccine for the initial stage. This number can be increased to 40 million doses per year by 2023 with the addition of production facilities.
Furthermore, the production capacity can be increased again to 100 million doses per year by 2024, depending on the needs and demands of the community.
Related news: Bio Farma's IndoVac vaccine obtains BPJPH's halal certificate
Related news: Govt redistributes COVID vaccines to assist regions with low stocks
Related news: AWcorna becomes first domestically produced mRNA COVID-19 vaccine
Translator: Andi Firdaus, Resinta S
Editor: Sri Haryati
Copyright © ANTARA 2022