The second phase of the clinical trial will kick off in October in Indonesia.Jakarta (ANTARA) - Indonesia is set to conduct the second phase of the clinical trial of the COVID-19 candidate vaccine produced by South Korean pharmaceutical company Genexine Inc. next month, Foreign Minister Retno Marsudi said.
“The second phase of the clinical trial will kick off in October in Indonesia,” she said following a working meeting with Commission I of the House of Representatives (DPR), here on Tuesday.
The first phase of the clinical trial of Genexine’s vaccine candidate, GX 19, has reached the final stages, she informed.
Genexine will collaborate with Indonesia’s PT Kalbe Farma Tbk to conduct the second phase of the trial in Indonesia.
In 2017, the two companies had established a joint-venture company called PT Kalbe Genexine Biologics (KGBio) to develop medicines and vaccines, among other things.
GX 19 has been developed by a consortium of companies, including Binex, International Vaccine Institute (IVI), GenNbio, KAIST, and POSTECH, with Genexine acting as a coordinator. The vaccine candidate has been developed from DNA material and has been designed to produce antigens and trigger an immune response in the body.
The South Korean Ministry of Food and Drug Security approved the first phase of the clinical trial of GX 19 in June this year.
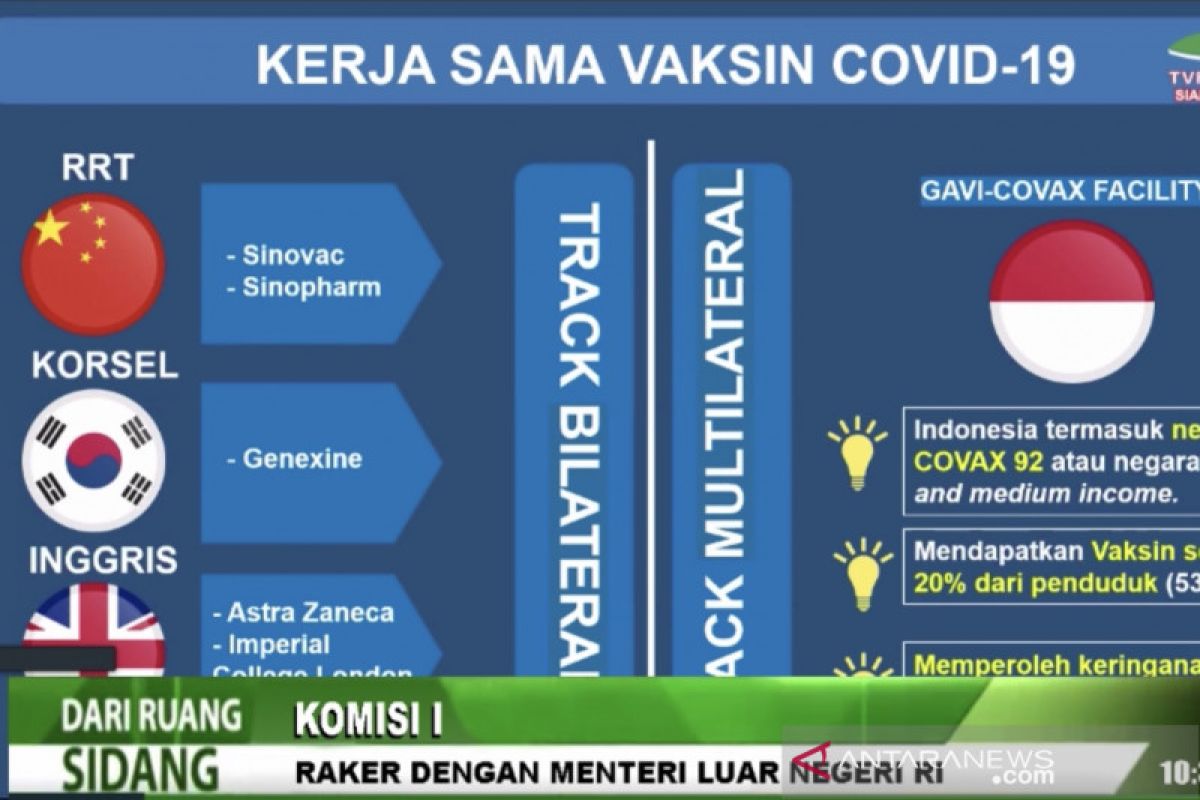
Indonesia is also collaborating with G42 Healthcare Holding of the United Arab Emirates for developing another COVID-19 vaccine. G42 has committed to providing 10 million doses of its COVID-19 candidate vaccine to Indonesia this year.
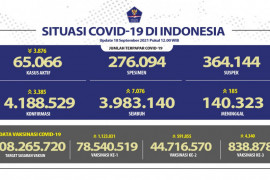
At the working meeting on Tuesday, Retno stated the government has secured 20-30 million doses of the COVID-19 candidate vaccine for 2020 and 290-340 million doses for 2021.
Besides Genexine, G42, Sinovac, and Sinopharm, she said the government is also looking into the possibility of cooperation with AstraZeneca and Imperial College London for vaccine development.
Bilateral cooperation for the development of COVID-19 vaccines is a short-term strategy to obtain safe COVID-19 vaccines quickly and at affordable prices, Retno explained.
"The second thing is long-term approaches to develop a national vaccine, called Red and White vaccine, that we hope will support self-reliance in vaccine production in Indonesia," she said.
Related news: Ministry maps flight routes for COVID-19 vaccine distribution
Related news: Indonesia fast tracks COVID-19 vaccine development
Translator: Genta Tenri M/Suharto
Editor: Rahmad Nasution
Copyright © ANTARA 2020