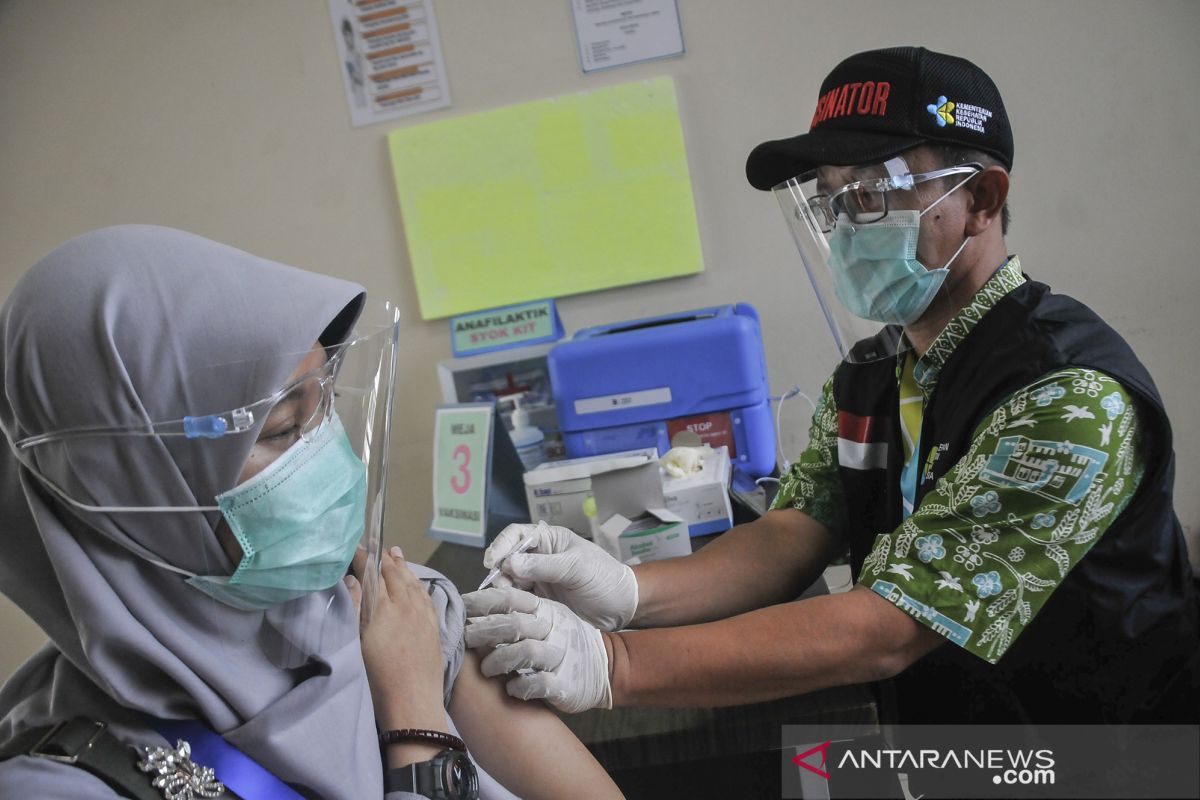
President Joko Widodo (Jokowi) paid a visit to Bogor, West Java on November 18, 2020 to review a vaccine simulation at a community health center (puskesmas). The next day, Vice President Ma'ruf Amin observed a similar simulation at a Bekasi puskesmas.
The simulations were carried out under strict observance of COVID-19 health protocols, such as maintaining distancing, washing hands, and wearing face masks.
The government is keen to begin its vaccination program in the third week of December, 2020. Over the past few months, it has been proactive in procuring COVID-19 vaccines from various sources through bilateral and multilateral cooperation.
The University of Pandjajaran and state-owned pharmaceutical company Bio Farma have been carrying out the final stage clinical trials of the Sinovac vaccine developed by China since August, and so far, the testing has run relatively smoothly.
Related news: COVID-19 vaccination simulations should be conducted optimally: Jokowi
Related news: President ensures COVID-19 vaccine for Indonesians listed under WHO
Meanwhile, President Widodo has said he is optimistic that COVID-19 vaccines would arrive in late November or December this year. They could be in the form of finished vaccines or raw materials to be processed at Bio Farma in Bandung.
He emphasized that the COVID-19 vaccination program must prioritize the safety and security of people.
Hence, all vaccines that are administered must be registered on the vaccine list of the World Health Organization (WHO). The vaccines are being purchased from companies, whose brands are registered with the WHO, he informed.
"I did not mention the brands, but they must be on the WHO list," he stated.
The government has signed an agreement to procure a total of 143 million doses of the COVID-19 vaccine concentrate from three Chinese pharmaceutical firms — Sinovac, Sinopharm, and CanSino, who will provide 65 million, 15 million, and 20 million doses of the vaccine concentrate, respectively.
In addition to China, Indonesia has also sought vaccines from United Arab Emirates (UAE) technology firm G-24, which agreed to send 10 million doses of its vaccine in mid-August through cooperation with state-run pharmaceutical firm PT Kimia Farma.
Indonesia has also procured 100 million doses of the COVID-19 vaccine developed by British-Swedish multinational pharmaceutical firm AstraZeneca, with the first delivery of the vaccine scheduled in the second quarter of 2021.
Related news: Jokowi emphesizes UN's role in facilitating vaccine access for all
After the vaccines arrive in Indonesia, they will undergo several stages of clearance at the Indonesian Agency of Drug and Food Control (BPOM) prior to being
allowed for use. Only after the BPOM clears them will they be used in the immunization program.
Jokowi spoke of his intention to again observe one or two other simulations to ensure that all aspects concerning the vaccination program are being thoroughly prepared.
The COVID-19 vaccination simulation is part of the government's endeavors to acclimatize the public to the COVID-19 vaccination plan and to offer a broad understanding of the importance of vaccine administration to break the chain of COVID-19 to end the pandemic.
Vice President Ma'ruf Amin recently said that pre-vaccination preparations comprise collection of data on vaccine recipients, stages of injection, distribution mechanism, and waste treatment model for the vaccines.
“[We are making] Several other preparations, include preparing data and names of people to be vaccinated throughout Indonesia, followed by the stages and distribution of vaccines as well as the apt approach to handle the large amount of vaccine waste," he stated.
The government has set a preliminary target of inoculating people in the 18-59 age group first. People aged over 59 years may likely get vaccinated later with other vaccine products.
Prioritized targets of COVID-19 vaccination include healthcare workers, security personnel, teachers, and other public service officers, who will be given vaccines for free.
Meanwhile, State-Owned Enterprises (BUMN) Minister Erick Thohir has appealed to Indonesians who are financially capable to pay for their own COVID-19 vaccine doses to help reduce the financial burden on the government due to the vaccination program.
Speaking at a webinar on "Preparing Infrastructure for COVID-19 Vaccination Data", Thohir said the government will issue two types of COVID-19 vaccines — one through the government-aided program and the other through the self-initiative vaccination program.
The government's aid-based vaccination program has been prepared for health workers and eligible members of communities, as revealed in data from the Workers Social Security Agency (BPJS Ketenagakerjaan) on assistance recipients, said Thohir, who is also deputy chair of the COVID-19 Handling and National Economic Recovery Committee (KPC-PEN).
Logistics is among the most crucial concerns in the vaccination program implementation, particularly for a country like Indonesia, the world's fourth most population nation and largest archipelagic country with over 17 thousand islands.
Logistics essentials for the vaccination drive include cold chain, a series of precisely coordinated events in a temperature-controlled environment to store, manage, and transport vaccines in order to deliver them to all parts of the world.
The Task Force for COVID-19 Response has said the cold chain preparation for the COVID-19 vaccine rollout in Indonesia has reached 97 percent completion.
Cold chain storage comprises refrigerators and freezers to store vaccines and carriers to distribute them to immunization centers, especially those located in open spaces, said Wiku Adisasmito, spokesman for the task force.
Vaccine storage would need special attention because a vaccine as a biological product is vulnerable to temperature change, hence it has to be stored at a certain temperature, which ranges from 2-8 degrees Celsius for freeze-sensitive vaccines to -15 to -25 degrees Celsius for heat-sensitive vaccines. Direct sun exposure can damage vaccines.
Currently, the government is preparing a list of regions that would be prioritized for the vaccination drive, based on the number of positive cases, population, a region's size, and other factors.
According to Health Ministry data, as of 2016, 78.8 percent of Public Health Centers (Puskesmas) have received refrigerators to store vaccines.
In 2018, the government ensured the setting up of cold chains in 9,951 Puskesmas across the country to support the implementation of immunization programs.
In the meantime, head of Indonesian Institute of Sciences (LIPI)'s Laboratory for Applied Genetic Engineering and Protein Design, Wien Kusharyoto, recently appealed to Indonesians to strictly observe the government-mandated health protocols while participating in the upcoming COVID-19 vaccination program.
The vaccination program would run for a long time owing to Indonesia's large population, he said. Besides, vaccine supplies may not become available and be distributed to all citizens at the same time, he pointed out.
Therefore, during the waiting period, health protocols must remain strictly implemented to break the chain of potential spread of the novel coronavirus disease in the community, he advised.
Implementing strict health protocols would remain important even after many Indonesians are vaccinated because it is yet to be determined how long antibodies remain in the body after a COVID-19 vaccine is administered, he pointed out.
Research has revealed that 66 percent of Indonesians are keen to get vaccinated, while 16 percent are reluctant to join the vaccination program.
As nearly 90 percent of Indonesia's population are Muslims, the government has asked the Indonesian Council of Ulemas (MUI) to resolve a matter regarding the guarantee that the vaccines are halal.
Meanwhile, Indonesia's Eijkman Institute for Molecular Biology has been developing an indigenous vaccine, named after the national flag, Red and White.
The Red and White vaccine is based on a recombinant protein sub-unit platform, and is expected to be ready for distribution at the end of 2021.
Related news: Red and White vaccine can be exported: Minister Brodjonegoro
Related news: Red and White vaccine may be distributed in Q4 2021
Editor: Sri Haryati
Copyright © ANTARA 2020