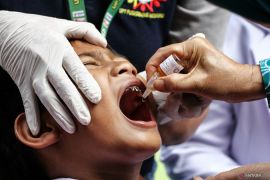
Supported by private and state-owned companies, the Gotong Royong program is seen as crucial for helping the government achieve the target of inoculating 181.5 million people to build herd immunity against the coronavirus.
Indonesia has been striving to win the fight against the COVID-19 pandemic since March last year. To stem the transmission of the lethal virus, the government has rolled out a nationwide vaccination program since January 13 this year.
Chairperson of the Indonesian Chamber of Commerce and Industry (Kadin), Rosan Perkasa Roeslani, said that at least 22,736 private companies have signed up for the program as of May 17, 2021.
Based on the Indonesian Health Ministry's Regulation No.10/2021 on the Gotong Royong Vaccination Scheme, all vaccine costs will be borne by participating companies under the program.
President Widodo said he is optimistic that once workers begin receiving the self-funded Gotong Royong vaccines, the number of vaccine recipients could reach 70 million by September, 2021.
"We expect to be able to vaccinate 70 million citizens by this August or no later than September (2021),” he said.
Widodo said he also hopes to see the COVID curve flattening during the August-September period and normalcy being restored in industrial productivity.
The first phase of the Gotong Royong Vaccination Program will be implemented in 18 companies.
In the first and second phase of the nationwide vaccination program, the government is targeting to cover 40,349,049 citizens, including human resources in the health sector, senior citizens, and public service workers.
According to the COVID-19 Handling Task Force, a total of 9,366,635 citizens have received two vaccine shots (have been fully vaccinated against COVID-19) and as many as 14,099,754 citizens have been administered the first vaccine shot as of May 20, 2021.
The government-funded vaccination program is using Sinovax vaccines bought from China and AstraZeneca vaccines received through the WHO-coordinated COVAX Facility.
Meanwhile, State-owned Enterprises (SOE) Minister Erick Thohir has said that his ministry has no plans to commercialize COVID-19 vaccines.
"Regarding prices, from the beginning, we in the SOE Ministry have been very open. We are not thinking about commercializing the COVID-19 vaccines," Thohir said at a press conference on May 19, 2021.
Related news: Single shot under Gotong Royong scheme to cost Rp500,000
Indonesia has so far secured 54 million vaccine doses free of cost through the World Health Organization (WHO) and spent nearly Rp77 trillion on procuring additional vaccine doses to cater to the large population of the country, which are being distributed free of charge, he disclosed.
For the self-funded program, the SOE Ministry and Kadin have kept vaccine prices transparent and they are being audited by the Financial and Development Supervisory Agency (BPKP), he added.
The Indonesian government has set the price for Sinopharm and CanSino vaccines that will be provided under the Gotong Royong Vaccination Program at Rp500 thousand per shot.
The price includes Rp375 thousand as the cost of each vaccine dose and Rp125 thousand as the cost of inoculation, Coordinating Minister for Economic Affairs, Airlangga Hartarto, informed at an online press conference originating from the Presidential Palace recently.
The government has signed a contract with Sinopharm for 7.5 million doses and with CanSino for five million vaccine doses, he said.
Indonesia's Food and Drug Control Agency (BPOM) has issued emergency-use authorization (EUA) for the COVID-19 vaccine manufactured by China's Sinopharm.
"Today, we have issued EUA for the vaccine produced by Beijing Bio-Institute Biological Products, which is a unit of Sinopharm, a subsidiary of the China National Biotech Group. This is the Sinopharm vaccine," BPOM head Penny Lukito stated during an online press conference on April 29, 2021.
On April 30, a total of 482,400 doses of the Sinopharm vaccine arrived in Jakarta from China, and on May 1, Indonesia was gifted 500 thousand doses of the Sinopharm vaccine by the United Arab Emirates. This June, Indonesia is expected to receive another one million doses as part of its order with Sinopharm.
From July to September this year, Indonesia is hoping to receive Moderna and Novavax vaccines to support the Gotong Royong vaccination program. It is also planning to allow Pfizer and Sputnix vaccines for the Gotong Royong program.
Earlier, the Health Ministry had appealed to people to not be picky about the COVID-19 vaccines offered by the government, saying the WHO has declared all currently authorized and recommended vaccines safe.
"So, there is no reason for the public to hesitate to participate in the vaccination program," Siti Nadia Tarmizi, spokesperson for the ministry, said .
The huge number of vaccine doses needed by Indonesia cannot be supplied by just one vaccine manufacturer, she said. In fact, countries around the world have been competing fiercely to secure vaccine supplies for their populations, she pointed out.
"At first, Indonesia received the Sinovac vaccine. Then, at the end of March and early April, 2021, we had the AstraZeneca vaccine, and in June or July, 2021, other vaccines are scheduled to arrive, (which) include Novavax and Pfizer," she noted.
The government is calling all vaccines as COVID-19 vaccines, so that they are no longer identified on the basis of producers, Tarmizi said.
Related news: Gotong Royong vaccination to cover 70 million by September: Jokowi
Editor: Rahmad Nasution
Copyright © ANTARA 2021