I think this is the hard work of our young human resources in creating a new vaccine from the upstream to the downstream, which took 1.5 years. It (the vaccine development) was silent, but eventually it resulted in IndoVacJakarta (ANTARA) -
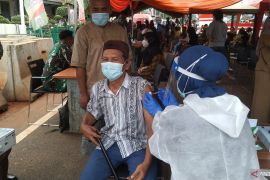
President Joko Widodo (Jokowi) on Thursday observed the first administration of the domestically produced COVID-19 vaccine, IndoVac, in Bandung, West Java.
“I think this is the hard work of our young human resources in creating a new vaccine from the upstream to the downstream, which took 1.5 years. It (the vaccine development) was silent, but eventually it resulted in IndoVac,” he said.
After arriving at the vaccination site, the President checked the packaging of the IndoVac vaccine. He then observed as the vaccine was administered by a health worker to a recipient.
Related news: Bio Farma's IndoVac vaccine obtains BPJPH's halal certificate
IndoVac uses a protein recombinant sub-unit technology platform produced by state pharmaceutical firm PT Bio Farma. Bio Farma conducted research and development (R&D) of the COVID-19 vaccine for almost a year from November 2021 to September 24, 2022.
The IndoVac vaccine received an emergency-use permit (EUA) from the Food and Drug Control Agency (BPOM) on September 24 for use as a primary vaccination (first and second doses) for adults or people aged above 18. Indovac also received halal certification from the Indonesian Ulema Council (MUI).
Bio Farma implemented good governance in the production of the vaccine, from the upstream to downstream, such as applying Good Manufacturing Practices (CPOB) and obtaining a CPOB certification from BPOM.
Bio Farma also carried out preclinical trials of the vaccine on animals. The Phase I clinical trial involved 175 animals, Phase II 360 animals, and Phase III 4,050 animals.
Related news: IndoVac vaccine is first COVID-19 vaccine produced in Indonesia: BPOM
The clinical trials showed that the IndoVac vaccine was safe in terms of adverse events following immunization (KIPI), with mild symptoms, such as pain in the injected area, reported.
IndoVac was also shown to have good efficacy in increasing the titer, or the concentration of antibodies.
During immuno-bridging trials with comparison vaccines with an efficacy of above 80 percent, the IndoVac vaccine was found to be non-inferior, which meant that it had better effectiveness compared to comparison vaccines, with an efficacy above 80 percent.
Related news: IndoVac injected first to unvaccinated residents
Related news: Jokowi launches domestically manufactured IndoVac COVID-19 vaccine
“I think this is the hard work of our young human resources in creating a new vaccine from the upstream to the downstream, which took 1.5 years. It (the vaccine development) was silent, but eventually it resulted in IndoVac,” he said.
After arriving at the vaccination site, the President checked the packaging of the IndoVac vaccine. He then observed as the vaccine was administered by a health worker to a recipient.
Related news: Bio Farma's IndoVac vaccine obtains BPJPH's halal certificate
IndoVac uses a protein recombinant sub-unit technology platform produced by state pharmaceutical firm PT Bio Farma. Bio Farma conducted research and development (R&D) of the COVID-19 vaccine for almost a year from November 2021 to September 24, 2022.
The IndoVac vaccine received an emergency-use permit (EUA) from the Food and Drug Control Agency (BPOM) on September 24 for use as a primary vaccination (first and second doses) for adults or people aged above 18. Indovac also received halal certification from the Indonesian Ulema Council (MUI).
Bio Farma implemented good governance in the production of the vaccine, from the upstream to downstream, such as applying Good Manufacturing Practices (CPOB) and obtaining a CPOB certification from BPOM.
Bio Farma also carried out preclinical trials of the vaccine on animals. The Phase I clinical trial involved 175 animals, Phase II 360 animals, and Phase III 4,050 animals.
Related news: IndoVac vaccine is first COVID-19 vaccine produced in Indonesia: BPOM
The clinical trials showed that the IndoVac vaccine was safe in terms of adverse events following immunization (KIPI), with mild symptoms, such as pain in the injected area, reported.
IndoVac was also shown to have good efficacy in increasing the titer, or the concentration of antibodies.
During immuno-bridging trials with comparison vaccines with an efficacy of above 80 percent, the IndoVac vaccine was found to be non-inferior, which meant that it had better effectiveness compared to comparison vaccines, with an efficacy above 80 percent.
Related news: IndoVac injected first to unvaccinated residents
Related news: Jokowi launches domestically manufactured IndoVac COVID-19 vaccine
Translator: Desca N, Kenzu T
Editor: Fardah Assegaf
Copyright © ANTARA 2022