Next-generation Sequencing-based CE-IVD Solution Provides Accurate, Reliable, Fast and Scalable End-to-end Genome-wide Noninvasive Prenatal Testing
Melbourne, Australia--(Antara/Business Wire)- Illumina, Inc., the global leader in DNA sequencing and array-based technologies and Next Generation Genomic Co., Ltd. (NGG Thailand), the Association of Southeast Asian Nations (ASEAN) leaders in laboratory services and reproductive science, today announced the launch of VeriSeq™ NIPT Solution v2 in Thailand, a CE-IVD, next-generation sequencing (NGS)-based approach to noninvasive prenatal testing (NIPT).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210531005030/en/
The automated in-lab IVD solution will allow NGG Thailand to launch the Qualifi Prenatal Test and be the first laboratory in South East Asia to detect anomalies that targeted assays miss and deliver more insights into the health of a pregnancy compared to standard NIPT offerings. Using Illumina’s VeriSeq NIPT Solution v2, the test delivers a comprehensive view of the fetal genome compared to other CE-IVD NIPT products, enabling healthcare providers to support expectant parents with informed, timely and personalized pregnancy management options better than ever before.
“With expanded NIPT, healthcare providers can obtain a comprehensive view of all 23 chromosome pairs while limiting risk to their patient,” said Assoc. Prof. Boonsri Chanrachakul, M.D., Ph.D., OB/GYN specializing in Maternal Fetal Medicine, Bangkok, Thailand. “This technique will offer the accurate, sensitive, and specific screening for chromosomal changes and provide more information to healthcare providers and expectant parents to empower their journey through pregnancy.”
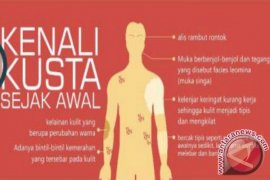
While conventional prenatal screening has been available for over 30 years, these tests were limited in their ability to screen beyond aneuploidies of chromosomes 21, 18, and 13. NGG Thailand’s Qualifi Prenatal Test uses VeriSeq NIPT Solution v2 to provide accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw. This noninvasive test provides a whole-genome sequencing (WGS) approach to NIPT, expanding prenatal screening beyond the three most common aneuploidies of chromosomes 21, 18 and 13, to all rare autosomal aneuploidies (RAAs), sex chromosome aneuploidies (SCAs), and large partial duplications and deletions.
Illumina Vice President and General Manager of Asia Pacific and Japan, Ms Gretchen Weightman, explained that the availability of VeriSeq NIPT Solution v2 is a key milestone in the partnership between Illumina and NGG Thailand.
“Illumina’s comprehensive technologies, coupled with NGG Thailand’s expertise, will enable healthcare providers and expectant parents to unlock the most critical information possible today,” Ms Weightman said.
The CE-IVD VeriSeq NIPT Solution v2 is now registered for use in Thailand, Vietnam, Singapore, South Korea, Australia, New Zealand, Israel, South Africa and across most countries in Europe.
Clinical Accuracy
The clinical accuracy of the CE-IVD VeriSeq NIPT Solution v2, with respect to outcomes determined by a clinical reference standard assessment, was demonstrated by evaluating more than 2,300 plasma samples from pregnant women with singleton and twin pregnancies undergoing prenatal screening for fetal chromosome aneuploidies and partial deletions and duplications of 7 Mb or greater. The study determined that VeriSeq NIPT Solution v2 provided highly sensitive and specific results – 98.8 percent passed assay quality control on the first pass.
Click here for more information on VeriSeq NIPT Solution v2.
ABOUT NEXT GENERATION GENOMIC THAILAND
Next Generation Genomic (NGG) is an excellent center for reproductive and prenatal genetic testing. Our mission is to provide high quality laboratory services with continuous quality improvement and leadership in education. Our services cover all steps from preconception to postnatal genetic testing. With our extensive experience and ISO15189/15190 certifications, we provide reliable, fast and high quality services for the ultimate goal of having healthy babies and families. To learn more, please visit our website: www.nggthailand.com and connect with us on Facebook.
ABOUT ILLUMINA
Illumina is improving human health by unlocking the power of the genome. Our focus on innovation has established us as the global leader in DNA sequencing and array-based technologies, serving customers in the research, clinical, and applied markets. Our products are used for applications in the life sciences, oncology, reproductive health, agriculture, and other emerging segments. To learn more, visit www.illumina.com and connect with us on Twitter, Facebook, LinkedIn, Instagram, and YouTube.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210531005030/en/
Contacts
Media:
Samantha Beal
US: +1 858.882.6843
ilmn-pr@illumina.comSource: Illumina, Inc.
Reporter: PR Wire
Editor: PR Wire
Copyright © ANTARA 2021