There were no additional cases of acute kidney failure in the last two weeksJakarta (ANTARA) - Health Ministry's spokesperson Mohammad Syahril confirmed that no new cases of acute kidney failure in children were reported in the last two weeks.
"There were no additional cases of acute kidney failure in the last two weeks," the spokesperson noted in a live broadcast on the Instagram account @ikatandokterindonesia on Friday.
Syahril remarked that out of the total of 324 cases of acute kidney failure, 200 patients had died and 111 patients recovered, while 13 patients were being treated.
The patients being treated have generally undergone long-term treatment for the disease that has entered the third stage, Syahril noted.
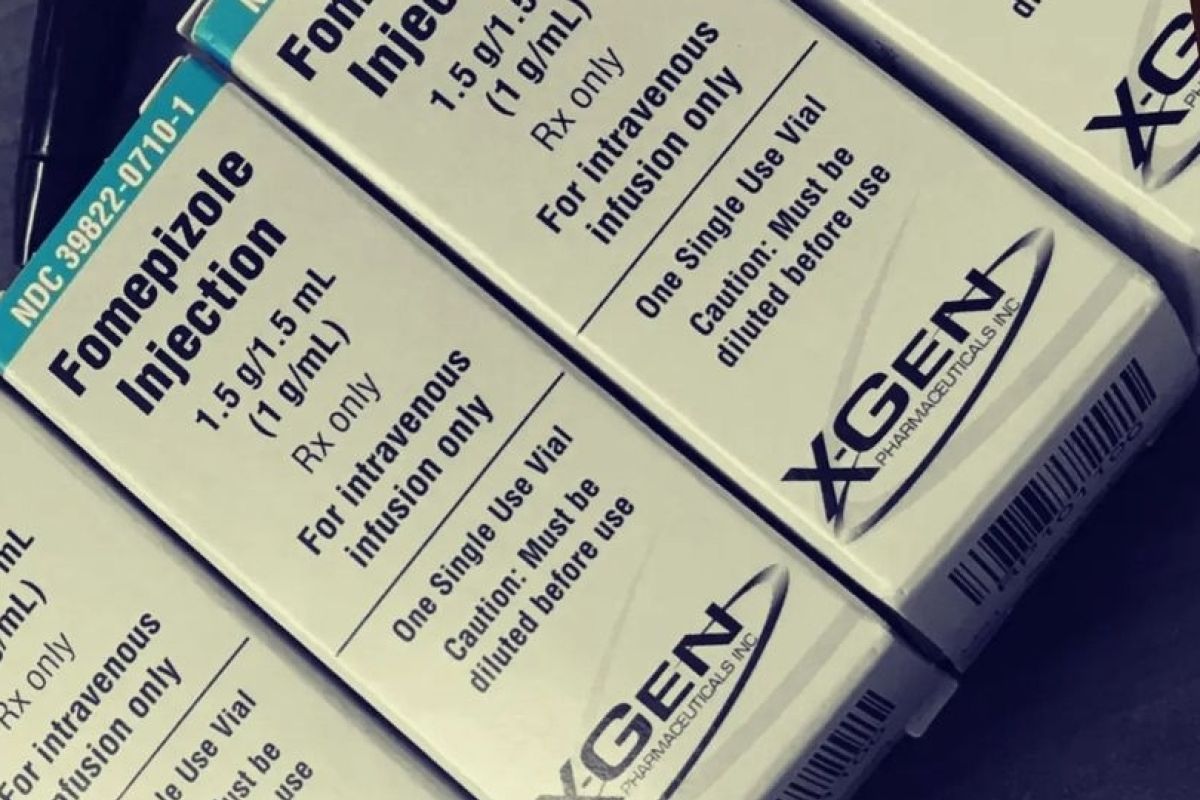
The World Health Organization had recommended the antidote Fomepizole to treat drug poisoning in patients with acute kidney failure.
Syahril remarked that the government had imported Fomepizole from Singapore to Indonesia. The drug has made a significant difference for patients with acute kidney failure whose disease was still in an early stage.
In addition, he urged the public to exercise caution and not carelessly administer medicine while handling a sick child in future.
"Many people used to immediately give medicine to their sick child and did not try the natural ways," he stated.
Earlier, the National Food and Drug Supervisory Agency (BPOM) published a list of syrup drugs that were initially banned but have been proven safe for consumption after being investigated. The list will continue to be updated from time to time since there are hundreds of syrup drugs circulating in Indonesia that must be investigated.
On Thursday, the BPOM announced an additional 168 syrup drug products that were declared free from dangerous chemicals -- Propylene Glycol, Polyethylene Glycol, Sorbitol, and Glycerin/Glycerol -- which were associated with acute kidney failure.
The BPOM has intensified surveillance over the quality of circulating medicinal syrup products and additional raw materials using the back-trace method as the development of supervision in distribution channels.
The Pharmaceutical Industry (IF) also independently conducted verification of the testing results of medicinal raw materials, including for ethylene glycol (EG) and diethylene glycol (DEG) contamination, in order to ensure the safety and quality of medicinal syrups.
This verification is conducted based on meeting the criteria, including supplier qualifications, raw material testing at each arrival and each container, and ensuring the test method follows the latest standards or pharmacopeia.
Related news: IDAI, RSCM conducting research on using ethanol as AKI antidote
Related news: Health Ministry open to pharmacopeia revision
Related news: No fortnightly increase in acute kidney injury cases: Health Ministry
Translator: Nanien Yuniar, Resinta S
Editor: Sri Haryati
Copyright © ANTARA 2022