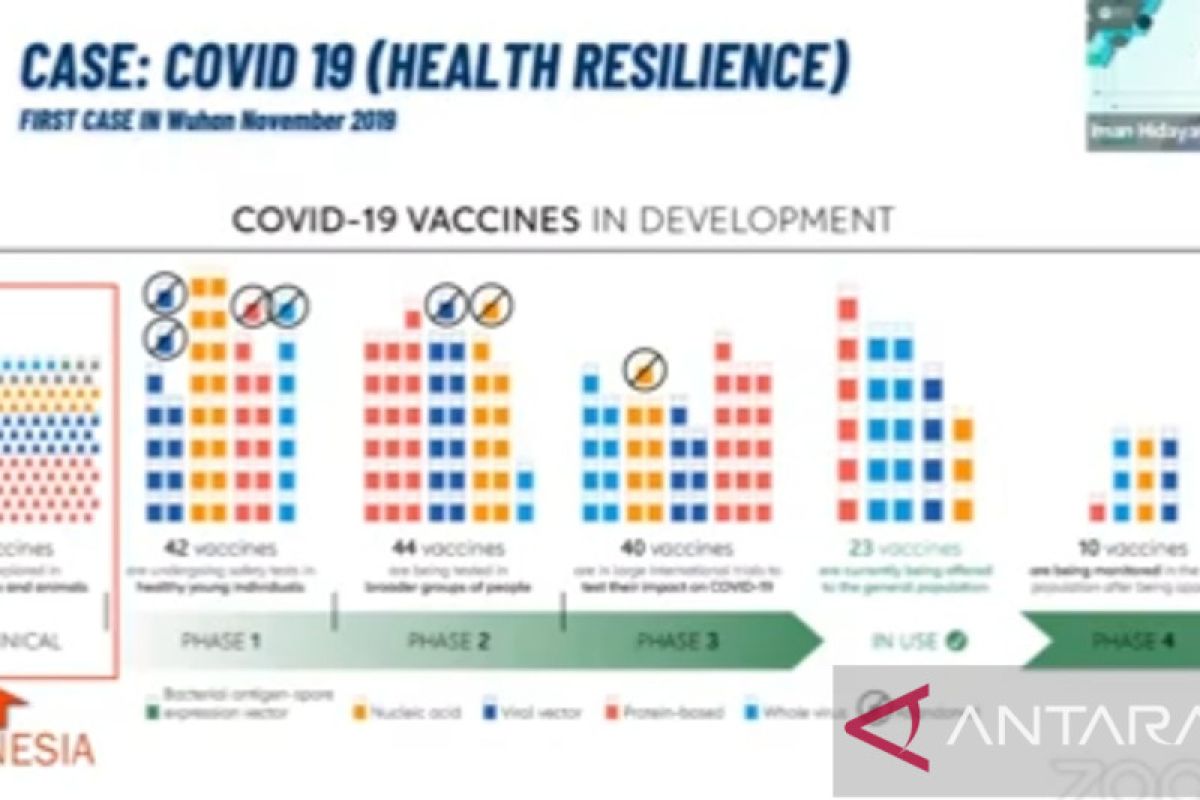
"Our current position is that we have become a part of the 194 vaccine candidates currently being developed in the entire world," BRIN's Life Science Research Organization Head Iman Hidayat said at an online event on Wednesday.
Indonesia's position is a part of efforts towards resolving the COVID-19 pandemic challenge that still plagues the world, he added.
However, unfortunately, it has been more than two years and Indonesia is still completing the clinical trials for the COVID-19 vaccine, he noted.
"We cannot be as fast as other nations such as China, the United States, and even India in developing vaccines," he said.
There are three problems that are hindering Indonesia's efforts to develop the Merah Putih vaccine, one of which is that Indonesia's researchers do not have experience developing a vaccine from scratch, he opined.
"When it comes to vaccines for polio and others that are produced by Bio Farma, we only purchase the license. We do not develop them from zero," he informed.
The next problem concerns industry collaboration, he said. This remains Indonesia's weak point because not many industries in Indonesia possess the necessary research and development facilities for developing vaccines, he noted.
Related news: EUA for Red and White vaccine likely in June: BPOM
The last problem relates to inadequate infrastructure, he said.
"These three bottlenecks are quite chronic in Indonesia, and have necessitated actions such as the development of several facilities by BRIN and facilitating researchers to expedite results," Hidayat said.
Currently, the Merah Putih vaccine is being developed by six institutions, he noted.
These institutions comprise the Eijkman Institute, Indonesian Institute of Sciences (LIPI), Bandung Institute of Technology (ITB), University of Indonesia (UI), Gadjah Mada University (UGM), and Airlangga University, who are using several platforms for developing the vaccine, he said.
Related news: Vaccine self-sufficiency crucial as measure against COVID-19 surge
A researcher from Eijkman, BRIN, Tedjo Sasmono noted that the Merah Putih vaccine that is being developed by his team is currently at the downstreaming stage with its industry partner, state-owned vaccine manufacturer PT Bio Farma.
He said he expects the pre-clinical and clinical trials to be carried out in the near future.
"Let us hope that the COVID-19 vaccine produced by our people would contribute to pandemic mitigation and become a platform for the nation's independence in vaccine research," he said.
Related news: Long wait for indigenous vaccine expected to end mid-year
Related news: Govt expedites COVID-19 vaccination to strengthen community's immunity
Translator: Zubi Mahrofi, Fadhli Ruhman
Editor: Fardah Assegaf
Copyright © ANTARA 2022